Understanding the influence of particle size on the reaction mechanism in porous La0.6Sr0.4CoO3-δ air electrodes
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
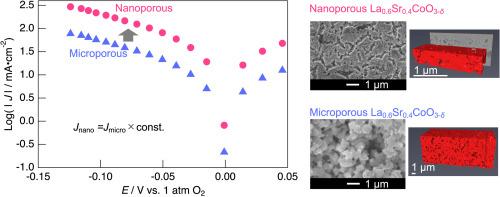
Enhancing the electrode performance of high-temperature devices such as solid oxide fuel cells and solid oxide electrolysis cells is essential for reducing their operating temperatures. One approach is to reduce the particle size in the porous electrodes, which has been shown to decrease their area specific resistance. In this study, we investigated the effect of particle size on the reaction mechanism of mixed-conducting La0.6Sr0.4CoO3-δ electrodes by fabricating half-cells with 650 nm-thick films composed of 55 ± 15 nm particles (nanoporous) and 15 μm-thick films composed of ∼800 nm particles (microporous). Electrochemical impedance spectroscopy and analysis using the Gerischer model were performed. For both micro- and nanoporous electrodes, a consistent relationship was found between the reaction field and the particle size, with the reaction field being about three times the particle size under our conditions. The surface reaction resistance and the effective oxygen transport resistance of microporous and nanoporous electrodes show overall similar values, with differences on the order of 0.5–1 orders of magnitude, indicating that they are largely comparable. In the DC polarization measurements, the current density of the microporous sample, when multiplied by a constant factor, closely matched that of the nanoporous sample. This suggests that the reaction mechanisms at both these electrodes were similar. This study demonstrates that the improvement in electrode performance with reduced particle size does not arise from intrinsic changes in the material properties, but rather from the increase in specific surface area. These findings provide useful guidelines for designing high-performance mixed-conducting porous electrodes.
了解粒径对La0.6Sr0.4CoO3-δ多孔空气电极反应机理的影响
提高固体氧化物燃料电池和固体氧化物电解电池等高温器件的电极性能对于降低其工作温度至关重要。一种方法是减小多孔电极中的颗粒尺寸,这已被证明可以降低其面积比电阻。在本研究中,我们通过制备由55±15 nm颗粒(纳米孔)组成的650 nm厚薄膜和由~ 800 nm颗粒(微孔)组成的15 μm厚薄膜的半电池,研究了粒径对混合导电La0.6Sr0.4CoO3-δ电极反应机理的影响。采用Gerischer模型进行了电化学阻抗谱和分析。对于微孔和纳米孔电极,反应场与粒径之间存在一致的关系,在我们的条件下,反应场约为粒径的三倍。微孔电极和纳米孔电极的表面反应电阻和有效氧输运电阻总体相近,差异在0.5-1数量级,具有很大的可比性。在直流极化测量中,微孔样品的电流密度,当乘以一个常数因子时,与纳米孔样品的电流密度非常接近。这表明这两个电极上的反应机制是相似的。该研究表明,电极性能的改善与减小颗粒尺寸不是由材料性能的内在变化引起的,而是由比表面积的增加引起的。这些发现为设计高性能混合导电多孔电极提供了有用的指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


