An interleukin-27-centered cytokine circuit regulates macrophage and T cell interactions in autoimmune diabetes
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
In the non-obese diabetic (NOD) mouse model of autoimmune diabetes, interleukin (IL)-27 stimulates interferon γ (IFNγ) production by CD4 and CD8 T cells and is essential for disease development. Here, we tested the role of IL-27 in cellular communication. Single-cell RNA sequencing and T cell adoptive transfer showed that IL-27 intrinsically controlled the differentiation of islet-infiltrating CD4 T cells by driving them toward an IL-21+ Th1 phenotype. Consequently, IL-27 signaling in CD4 T cells was important for BATF and granzyme B expression in islet CD8 T effectors. BATF overexpression increased the diabetogenic potential of β cell autoreactive CD8 T cells lacking help from CD4 T cell-derived IL-21. Macrophages were the main source of IL-27 in the islets, whose expression correlated with T cell infiltration. IFNγ and CD40 signaling conferred by activated T cells induced macrophage IL-27 production. Collectively, our findings reveal a role for IL-27 in orchestrating interconnected positive feedback loops involving CD4 T cells, CD8 T cells, and macrophages in autoimmune diabetes.
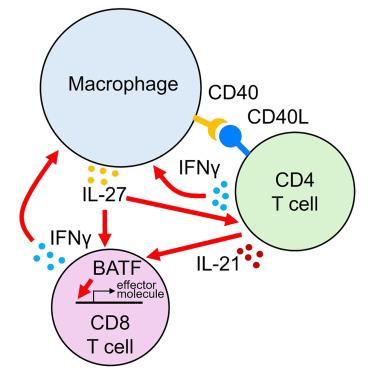
以白细胞介素27为中心的细胞因子回路调节自身免疫性糖尿病中巨噬细胞和T细胞的相互作用
在自身免疫性糖尿病的非肥胖糖尿病(NOD)小鼠模型中,白细胞介素(IL)-27刺激CD4和CD8 T细胞产生干扰素γ (IFNγ),是疾病发展所必需的。在这里,我们测试了IL-27在细胞通讯中的作用。单细胞RNA测序和T细胞过继转移表明,IL-27通过驱动浸润胰岛的CD4 T细胞向IL-21+ Th1表型发展,内在地控制着它们的分化。因此,CD4 T细胞中的IL-27信号对于胰岛CD8 T效应物中BATF和颗粒酶B的表达是重要的。BATF过表达增加了缺乏CD4 T细胞来源IL-21帮助的β细胞自身反应性CD8 T细胞的致糖尿病潜能。巨噬细胞是胰岛IL-27的主要来源,其表达与T细胞浸润相关。活化T细胞传递的IFNγ和CD40信号诱导巨噬细胞产生IL-27。总的来说,我们的研究结果揭示了IL-27在自身免疫性糖尿病中协调CD4 T细胞、CD8 T细胞和巨噬细胞相互关联的正反馈回路中的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


