Macrophage repolarization by immune checkpoint blockade drives T cell engagement in the tumor microenvironment
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Immunotherapy combinations can improve patient outcomes, yet the interactions within the tumor microenvironment (TME) that drive therapeutic synergy are poorly understood. Tumor establishment drives monocyte recruitment and differentiation into tumor-associated macrophages (TAMs), which have essential roles in coordinating immune responses and are thus attractive targets for therapeutic modulation. In a murine model of combination anti-programmed cell death protein 1 (PD-1) and its ligand (anti-PD-L1) checkpoint blockade, tumor control was associated with increased infiltration of CD8+ T cells and M1-like repolarization of TAMs. Live-cell imaging of the tumor microenvironment revealed close contacts between tumor-infiltrating CD8+ T cells and TAMs, in which the extent of the contact interfaces increased with combination immunotherapy. Treatment with anti-PD-L1 was able to increase macrophage expression of pro-inflammatory factors and phagocytic activity, suggesting a role for TAMs in reactivating CD8+ T cells in the TME. However, co-treatment with anti-PD-1 was ultimately necessary for tumor control, indicating the need for combination targeting of the TME.
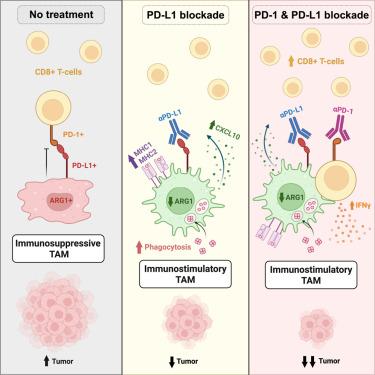
免疫检查点阻断巨噬细胞复极化驱动T细胞参与肿瘤微环境
免疫治疗组合可以改善患者的预后,但肿瘤微环境(TME)内驱动治疗协同作用的相互作用尚不清楚。肿瘤形成驱动单核细胞募集和分化为肿瘤相关巨噬细胞(tam),它们在协调免疫反应中起重要作用,因此是治疗调节的有吸引力的靶点。在联合抗程序性细胞死亡蛋白1 (PD-1)及其配体(抗pd - l1)检查点阻断的小鼠模型中,肿瘤控制与CD8+ T细胞浸润增加和tam的m1样复极化有关。肿瘤微环境的活细胞成像显示肿瘤浸润性CD8+ T细胞与tam之间的密切接触,其中接触界面的程度随着联合免疫治疗而增加。抗pd - l1治疗能够增加巨噬细胞促炎因子的表达和吞噬活性,提示tam在TME中重新激活CD8+ T细胞的作用。然而,与抗pd -1联合治疗最终是肿瘤控制所必需的,这表明需要联合靶向TME。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


