Liquiritin in Chaihu-shugan-san alleviates Parkinson's Disease via PPARα-mediated inhibition of SCD1/FADS2-dependent Ferroptosis
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
Parkinson's disease (PD) is a progressive neurodegenerative disorder with increasing global prevalence. Chaihu-shugan-san (CSS) is a Chinese medicine compound with reported neuroprotective properties. However, the precise bioactive constituents and underlying mechanisms of CSS in the context of PD remain poorly defined.
Purpose
This study aimed to systematically evaluate the therapeutic potential of CSS, characterize its bioactive constituents, and elucidate the potential molecular mechanisms in PD.
Methods
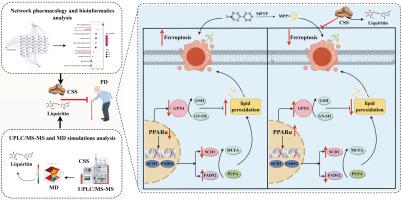
A network pharmacology-bioinformatics framework was established to predict the potential targets of CSS in PD. MPTP-induced PD mouse models were validated by behavioral tests, histopathology (HE staining), immunohistochemistry (IHC), and Western blot (WB). Machine learning and ROC curve analysis pinpointed key ferroptosis-related genes linked to CSS and PD, with functional enrichment highlighting possible pathways. Further validation was performed using IHC, GPx, MDA, and WB assays. UPLC-MS/MS was employed to characterize bioactive CSS components, followed by druggability screening, molecular docking, and molecular dynamics (MD) simulations. In vitro, MES23.5 cells exposed to MPP⁺ were used to evaluate the neuroprotective effects of Liquiritin (LIQ), the principal active compound in CSS, via CCK-8, LDH, oxidative stress markers (GPx, MDA), lipid peroxidation (C11-BODIPY), iron accumulation (FerroOrange), and WB. Additional validation of ferroptosis modulation was performed in vivo and in vitro.
Results
Multidisciplinary investigations employing prediction and experimental validation consistently demonstrated the neuroprotective effects of CSS against MPTP-induced PD mouse. Machine learning, ROC curve analysis and in vivo validation identified CSS ameliorates PD by inhibiting ferroptosis through PPARα activation. Intriguingly, CSS exhibited therapeutic potential by counteracting ferroptosis-triggered PD-like pathological manifestations in mice. Integrated analysis involving UPLC-MS/MS, druggability screening, molecular docking and MD simulations demonstrated LIQ to be a core bioactive compound in CSS. Mechanistic studies revealed that LIQ protects against MPP+- or erastin-induced ferroptosis in MES23.5 cells via activation of the PPARα/SCD1/FADS2 pathway. Pharmacological inhibition of PPARα abolished LIQ's protective effects against both ferroptosis and PD progression, definitively establishing PPARα activation as essential for LIQ-mediated neuroprotection.
Conclusion
LIQ, a major bioactive component of CSS, ameliorates PD pathology by inhibiting ferroptosis through activation of the PPARα/SCD1/FADS2 signaling pathway. These findings uncover a novel anti-ferroptosis mechanism and provide a promising therapeutic framework for the prevention and treatment of PD.
柴虎舒肝散中的利尿素通过ppar α介导的抑制SCD1/ fads2依赖性铁凋亡来缓解帕金森病
帕金森氏病(PD)是一种进行性神经退行性疾病,全球患病率不断上升。柴胡疏肝散(CSS)是一种具有神经保护作用的中药复方。然而,在PD的背景下,CSS的确切生物活性成分和潜在机制仍然不清楚。目的本研究旨在系统评价CSS的治疗潜力,表征其生物活性成分,并阐明其在PD中的潜在分子机制。方法建立网络药理学-生物信息学框架,预测CSS在PD中的潜在靶点。通过行为学实验、组织病理学(HE染色)、免疫组化(IHC)和Western blot (WB)验证mptp诱导的PD小鼠模型。机器学习和ROC曲线分析确定了与CSS和PD相关的关键铁衰相关基因,功能富集突出了可能的途径。通过IHC、GPx、MDA和WB检测进一步验证。采用UPLC-MS/MS对CSS组分进行生物活性表征,然后进行药物筛选、分子对接和分子动力学(MD)模拟。在体外,使用暴露于MPP +的MES23.5细胞,通过CCK-8、LDH、氧化应激标志物(GPx、MDA)、脂质过氧化(C11-BODIPY)、铁积累(FerroOrange)和WB来评估CSS中主要活性化合物LIQ的神经保护作用。进一步验证了铁下垂调节在体内和体外进行。结果采用预测和实验验证的多学科研究一致证明了CSS对mptp诱导的PD小鼠的神经保护作用。机器学习、ROC曲线分析和体内验证表明,CSS通过PPARα激活抑制铁下垂来改善PD。有趣的是,CSS通过在小鼠中对抗铁中毒引发的pd样病理表现显示出治疗潜力。UPLC-MS/MS、药物筛选、分子对接和MD模拟等综合分析表明,LIQ是CSS的核心生物活性化合物。机制研究表明,LIQ通过激活PPARα/SCD1/FADS2通路,可防止MPP+-或erastin诱导的MES23.5细胞铁凋亡。药理抑制PPARα可消除LIQ对铁下沉和PD进展的保护作用,从而明确确立了PPARα激活对LIQ介导的神经保护至关重要。结论liq是CSS的主要生物活性成分,通过激活PPARα/SCD1/FADS2信号通路抑制铁下垂,改善PD病理。这些发现揭示了一种新的抗铁下垂机制,为PD的预防和治疗提供了一个有希望的治疗框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


