Neutron radiography analysis of water management in a passive proton-exchange membrane fuel cell with superhydrophobic catalyst layers
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Water transport in proton-exchange membrane fuel cells (PEMFCs) with superhydrophobic catalyst layers (CLs) has been studied with neutron radiography. Superhydrophobic CLs were deposited by electrospray on the membrane to be tested on the cathode and anode sides of the cells. The cells are operated under passive conditions without applying convective forces for gas inlets. Operando water thickness images show that electrosprayed CLs accelerate water elimination by natural forces. Water management shows three stages characterized by different transport processes: 1) surface diffusion of bound water (associated to the ionomer), followed by 2) free water transport (liquid phase) by capillary diffusion towards the gas diffusion layer pores, and 3) evacuation of free water from the surface of the cathode by natural forces. A vertical cell, with cell plane parallel to gravity field, favors the action of evaporation and the dragging of water drops and slugs over the cathode surface leading to higher passive performance during the second and third stages. In this orientation, a superhydrophobic CL on one electrode and a hydrophilic CL on the other doubles the power generation with respect to a standard cell. In the horizontal position, superhydrophobic CLs can alleviate transport hindrances due to less effective natural forces.
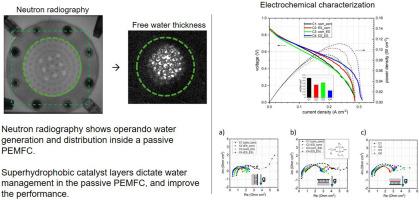
具有超疏水催化剂层的被动质子交换膜燃料电池水管理的中子照相分析
用中子射线照相技术研究了超疏水催化剂层质子交换膜燃料电池(pemfc)中的水输运。超疏水CLs通过电喷雾沉积在电池正极两侧的待测膜上。电池在被动条件下运行,没有对气体进口施加对流力。Operando水厚度图像显示,电喷涂CLs通过自然力加速了水的消除。水的管理表现为三个阶段,以不同的传输过程为特征:1)结合水(与离聚体相关)的表面扩散,随后2)自由水(液相)通过毛细扩散向气体扩散层孔隙的传输,以及3)自由水通过自然力从阴极表面排出。垂直电池,电池平面与重力场平行,有利于蒸发作用和水滴和段塞在阴极表面的拖拽作用,从而在第二和第三阶段获得更高的被动性能。在这个方向上,一个电极上的超疏水氯离子和另一个电极上的亲水氯离子的发电量是标准电池的两倍。在水平位置,超疏水CLs可以减轻由于不太有效的自然力的运输障碍。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


