Dynamic regulation of ionomer/catalyst interface via alcohol coordination engineering: Toward high–efficiency proton and oxygen transport in proton exchange membrane fuel cells
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
To bolster the proliferation of proton exchange membrane fuel cells (PEMFCs), the development of high–performance, low–platinum PEMFCs is imperative. Based on interface alcohol coordination engineering, this study systematically investigates the structure–activity relationships of alcohol additives and their impact on the ionomer/catalyst interface from microscopic interactions to macroscopic catalyst layer (CL) changes. The findings reveal that the alcohol engineering modulates the ionomer/catalyst interface distribution through the coordination with −SO3H groups, thus improving the O2 and proton transport capacity of the CL. Tert–heptanol, with its most alkaline structure, forms the strongest coordination with ionomers, resulting in the most notable improvements in a 25.23 % increase in peak power density under H2–Air conditions. In the optimized tert–heptanol system, peak power densities reach 0.93 W cm−2 under H2–Air and 2.49 W cm−2 under H2–O2, with an impressively low O2 transport resistance of 10.9 s m−1. These insights will inform future ionomer/catalyst interface designs for PEMFCs and related technologies.
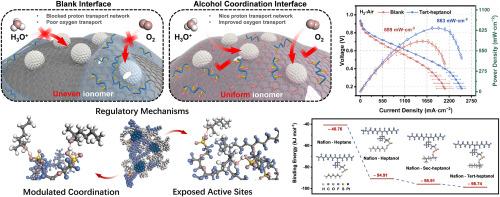
醇配位工程对离子/催化剂界面的动态调控:迈向质子交换膜燃料电池中高效的质子和氧传输
为了促进质子交换膜燃料电池(pemfc)的发展,开发高性能、低铂的质子交换膜燃料电池势在必行。本研究基于界面醇配位工程,从微观相互作用到宏观催化剂层(CL)的变化,系统研究了醇类添加剂的构效关系及其对离聚物/催化剂界面的影响。研究结果表明,醇工程通过与−SO3H基团的配位调节了离聚物/催化剂的界面分布,从而提高了CL的O2和质子传输能力。叔庚醇具有最碱性的结构,与离聚物形成最强的配位,在H2-Air条件下,其峰值功率密度提高了25.23%,效果最为显著。优化后的三庚醇体系在H2-Air和H2-O2条件下的峰值功率密度分别达到0.93 W cm−2和2.49 W cm−2,O2输运阻力极低,为10.9 s m−1。这些见解将为未来pemfc的离子/催化剂界面设计和相关技术提供信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


