Dual‑salt electrolyte design enabled by synergistic solvation and interfacial regulation for fast charging of lithium‑ion batteries
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
To address the performance limitations of conventional LiPF6-carbonate electrolytes under extreme temperatures and high-rate charging, lithium difluoro(oxalato)borate (LiDFOB) is introduced into the LiPF6-carbonate electrolyte to form a dual-salt system. The optimization mechanism enhancing the fast-charging capability of LiNi0.52Co0.2Mn0.28O2 (NCM523) cathode is systematically explored. Molecular dynamics simulations and electrochemical characterization demonstrate the reconstruction of Li+ solvation structures, expanding the voltage window and reducting Li+ desolvation barriers. In addition, the incorporation of LiDFOB induces the generation of a LiF/LixBOyFz-enriched cathode-electrolyte interphase, which effectively suppresses the dissolution of transition metals. In situ impedance measurements reveal the accelerated interfacial charge transfer kinetics. As expected, the NCM523 cathode achieves an 82 % state-of-charge (SOC) in 12 min at 5 C (25 °C) with 87 % capacity retention after 100 cycles, and exhibits a 65 % higher discharge capacity at 1 C than the baseline at −20 °C. The 1 Ah pouch cells based on LiNi0.52Co0.2Mn0.28O2 cathodes, graphite anodes, and 0.5 wt% LiDFOB-modified electrolyte demonstrate fast-charging capabilities: charging 97 % of the pouch cell capacity within 30 min (2 C) and 80 % within 15 min (4 C) at 25 °C. This study offers a practical electrolyte design strategy that enhances the fast-charging performance of lithium-ion batteries (LIBs) over a wide temperature range (from −20 to 25 °C).
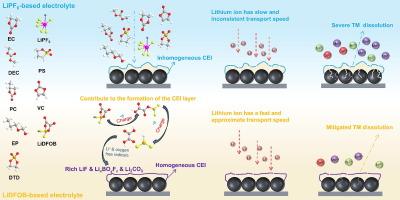
双盐电解质设计,通过协同溶剂化和界面调节实现锂离子电池的快速充电
为了解决传统lipf6 -碳酸盐岩电解质在极端温度和高倍率充电条件下的性能限制,将二氟(草酸)硼酸锂(LiDFOB)引入lipf6 -碳酸盐岩电解质中,形成双盐体系。系统探索了提高LiNi0.52Co0.2Mn0.28O2 (NCM523)阴极快速充电性能的优化机理。分子动力学模拟和电化学表征证明了Li+溶剂化结构的重建,扩大了电压窗,降低了Li+脱溶障碍。此外,LiDFOB的掺入诱导了LiF/ lixboyfz富集的阴极-电解质界面的产生,有效抑制了过渡金属的溶解。原位阻抗测量揭示了加速的界面电荷转移动力学。正如预期的那样,NCM523阴极在5℃(25℃)下在12分钟内达到82%的荷电状态(SOC), 100次循环后容量保持率为87%,在1℃时的放电容量比在- 20℃时的基线放电容量高65%。基于LiNi0.52Co0.2Mn0.28O2阴极、石墨阳极和0.5 wt% lidfob修饰电解质的1 Ah袋电池显示出快速充电能力:在25°C下,30分钟(2℃)充电97%,15分钟(4℃)充电80%。这项研究提供了一种实用的电解质设计策略,可以提高锂离子电池(lib)在宽温度范围内(从- 20到25°C)的快速充电性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


