Solvation-interphase synergistic regulation empowering high-temperature and fast-charging lithium metal batteries
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
Lithium metal batteries (LMBs) have emerged as pivotal energy storage solutions for electric vehicles and portable electronics. However, their operation under extreme conditions (high-temperature and fast-charging conditions) faces significant challenges, including accelerated electrolyte decomposition, interfacial instability, and potential thermal runaway risks. To address these challenges, we present a solvation-interphase synergistic regulation strategy using 2-fluorobenzenesulfonamide (2-FBS) as a multifunctional electrolyte additive. The 2-FBS molecule effectively modulates the Li+ solvation structure by reducing the coordination of ethylene carbonate (EC) solvent. This transformation suppresses EC-induced parasitic reactions while scavenging superoxide radicals, thereby mitigating gas evolution at electrode interfaces. Upon preferential decomposition, 2-FBS further promotes the formation of a robust LiF-Li3N-Li2S-rich interphase with exceptional mechanical strength (Young’s modulus: 39.4 GPa). This inorganic-rich hybrid interphase simultaneously enables dendrite-free lithium plating and enhances cathode thermal stability. Consequently, 2-FBS-modified electrolyte empowers LiCoO2//Li cells to deliver 82.8 % capacity retention after 800 cycles at 55 °C and sustain 81.2 % capacity retention after 1500 cycles at 4 C. Moreover, practical validation through nail penetration tests confirms the effectiveness of the electrolyte in preventing thermal propagation in fully charged pouch cells. This work establishes a paradigm for enabling reliable battery operation under extreme conditions through synergistic solvation and interphase engineering.
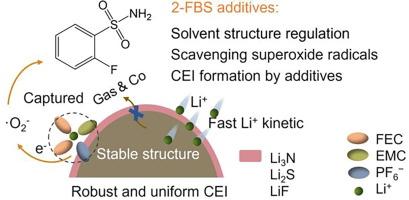
溶剂-相间协同调节增强高温快速充电锂金属电池
锂金属电池(lmb)已成为电动汽车和便携式电子产品的关键储能解决方案。然而,它们在极端条件下(高温和快速充电条件下)的运行面临着重大挑战,包括加速电解质分解、界面不稳定和潜在的热失控风险。为了解决这些挑战,我们提出了一种溶剂-相间协同调节策略,使用2-氟苯磺酰胺(2-FBS)作为多功能电解质添加剂。2-FBS分子通过降低碳酸乙烯(EC)溶剂的配位,有效调节Li+溶剂化结构。这种转化抑制了ec诱导的寄生反应,同时清除了超氧自由基,从而减轻了电极界面的气体演化。经过优先分解,2-FBS进一步促进了具有优异机械强度(杨氏模量:39.4 GPa)的富life - li3n - li2s界面相的形成。这种富含无机的杂化界面同时使无枝晶的锂电镀成为可能,并提高了阴极的热稳定性。因此,2- fbs修饰的电解质使LiCoO2//Li电池在55°C下循环800次后容量保持率为82.8%,在4°C下循环1500次后容量保持率为81.2%。此外,通过指甲穿透测试的实际验证证实了电解质在完全充电的袋状电池中防止热传播的有效性。这项工作建立了一个范例,通过协同溶剂化和间相工程,在极端条件下实现可靠的电池运行。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


