Dipole-induced built-in electric field manipulation for regulating Zn electrodeposition topology in high-performance aqueous Zn ion storage
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
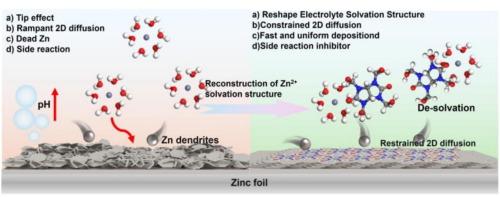
Aqueous Zn-ion storage offers high capacity and safety, but practical use is hindered by dendrite formation, side reactions, and hydrogen evolution, affecting stability and efficiency. Herein, tetramethylol acetylenediurea (TA) is proposed as an effective electrolyte additive that modulates the Zn2+ deposition environment via coordination competition. The polar functional groups of TA restructure the solvation sheath, while its molecular dipoles generate localized electric fields that accelerate Zn2+ migration and promote directional (002)-oriented deposition. These effects collectively suppress side reactions and enhance Zn plating/stripping reversibility. The four hydroxyl (–OH) and conjugated ketone groups (C=O) in the TA molecule have strong coordination ability (Lewis basicity) and can form a stable [Zn(TA)(H2O)n]2+ with Zn2+, reducing the number of free water molecules and the proportion of active water in the solvation sheath. The TA molecules are adsorbed onto the Zn anode surface, leading to the redistribution of the local spatial electric field and homogenization of ion flux dynamics. Its conjugated planar structure can induce Zn2+ to preferentially deposit along the (002) crystal plane. Zn//Zn symmetric cell using TA-containing ZnSO4 electrolyte exhibits stable cycling for more than 2240 h at 1 mA cm−2, 1 mAh cm−2. The Zn//activated carbon (AC) full-cell can stably cycle 30,000 cycles at 5 A g−1 with a capacity retention rate of 90 %. This study provides important insights into electrolyte engineering strategies for stabilizing Zn anodes, highlighting the potential of molecular design additives in next-generation Zn2+ energy storage systems.
偶极感应内建电场操纵调节高性能水锌离子存储中锌电沉积拓扑结构
水溶液锌离子存储具有高容量和安全性,但实际应用受到枝晶形成、副反应和析氢的阻碍,影响了稳定性和效率。本文提出了四甲基乙二脲(tetramethylol acetylenediurea, TA)作为一种有效的电解质添加剂,通过配位竞争调节Zn2+沉积环境。TA的极性官能团重构了溶剂化鞘,而其分子偶极子产生了局域电场,加速了Zn2+的迁移,促进了定向(002)沉积。这些效应共同抑制副反应,提高锌电镀/剥离的可逆性。TA分子中的4个羟基(-OH)和共轭酮基(C=O)具有较强的配位能力(Lewis碱度),可与Zn2+形成稳定的[Zn(TA)(H2O)n]2+,减少了游离水分子的数量和溶剂化鞘中活性水的比例。TA分子被吸附在Zn阳极表面,导致局部空间电场的重新分布和离子通量动力学的均匀化。其共轭平面结构可诱导Zn2+沿(002)晶面优先沉积。使用含ta的ZnSO4电解质的Zn//Zn对称电池在1ma cm - 2和1mah cm - 2下稳定循环2240 h以上。锌//活性炭(AC)全电池在5 A g−1下可稳定循环30000次,容量保持率达90%。该研究为稳定Zn阳极的电解质工程策略提供了重要见解,突出了分子设计添加剂在下一代Zn2+储能系统中的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


