Preparation of magnesium phosphate cement by salt lake magnesium slag and its hydration process
IF 3.7
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Magnesium phosphate cements (MPC) have shown promising applications in many fields, but high raw material prices hinder their development. The production of salt lake MPC (SLMPC) from magnesium slag (MS), a byproduct of lithium extraction from salt lakes, offers significant environmental and economic advantages. In this study, a low-cost magnesia raw material was obtained through the calcination of MS, which was subsequently utilized in conjunction with KH2PO4 to prepare SLMPC. The changes in hydration products, microscopic morphology, solution pH value, and TG content during the SLMPC curing process, and the hydration kinetics equation and model were used to study the hydration processes of SLMPC. The results show that the outcome indicates that the SLMPC system entered the accelerated reaction stage within 6 min after mixing, where the highest heat release rate was 6.29 J·g−1·min−1, the maximum heat release was 205.3 J·g−1, and the main hydration product appeared at 50–60 min. The hydration behavior of SLMPC exhibits similarities to that of traditional MPC. Specifically, the acceleration phase is governed by an autocatalytic reaction, the deceleration phase is influenced by both autocatalytic reactions and diffusion processes, and the stabilization phase is predominantly controlled by diffusion mechanisms. This paper aims to establish the theoretical foundation for the industrial application of MS and the cost-effective production of MPC.
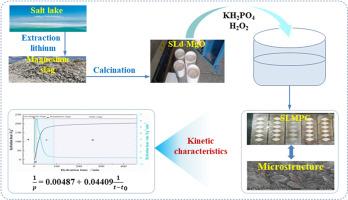
盐湖镁渣制备磷酸镁水泥及其水化工艺研究
磷酸镁胶结剂(MPC)在许多领域显示出良好的应用前景,但高昂的原材料价格阻碍了其发展。利用盐湖提锂的副产物镁渣(MS)生产盐湖MPC (SLMPC)具有显著的环境和经济优势。在本研究中,通过MS煅烧获得了一种低成本的氧化镁原料,随后将其与KH2PO4结合用于制备SLMPC。利用SLMPC固化过程中水化产物、微观形貌、溶液pH值和TG含量的变化,以及水化动力学方程和模型研究了SLMPC的水化过程。结果表明:混合后6 min内,SLMPC体系进入加速反应阶段,最大放热速率为6.29 J·g−1·min−1,最大放热速率为205.3 J·g−1,主要水化产物出现在50 ~ 60 min;SLMPC的水化行为与传统MPC相似。其中,加速阶段受自催化反应控制,减速阶段受自催化反应和扩散过程共同影响,稳定阶段主要受扩散机制控制。本文旨在为MS的工业应用和MPC的高性价比生产奠定理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Journal of Chemical Engineering
工程技术-工程:化工
CiteScore
6.60
自引率
5.30%
发文量
4309
审稿时长
31 days
期刊介绍:
The Chinese Journal of Chemical Engineering (Monthly, started in 1982) is the official journal of the Chemical Industry and Engineering Society of China and published by the Chemical Industry Press Co. Ltd. The aim of the journal is to develop the international exchange of scientific and technical information in the field of chemical engineering. It publishes original research papers that cover the major advancements and achievements in chemical engineering in China as well as some articles from overseas contributors.
The topics of journal include chemical engineering, chemical technology, biochemical engineering, energy and environmental engineering and other relevant fields. Papers are published on the basis of their relevance to theoretical research, practical application or potential uses in the industry as Research Papers, Communications, Reviews and Perspectives. Prominent domestic and overseas chemical experts and scholars have been invited to form an International Advisory Board and the Editorial Committee. It enjoys recognition among Chinese academia and industry as a reliable source of information of what is going on in chemical engineering research, both domestic and abroad.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


