Exploring the structural and conductivity behaviours of mechanochemically and hydrothermally synthesized Ce3+-doped BaSnF4 solid electrolytes for all-solid-state fluoride-ion batteries
IF 3.3
4区 材料科学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
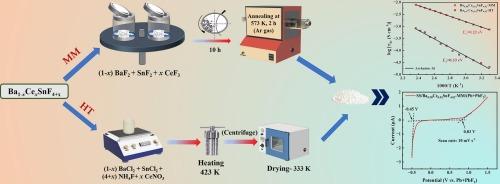
Recent studies on batteries have drawn significant attention to all-solid-state fluoride-ion batteries (FIBs) as potential alternatives to the conventional Li-ion batteries because of their higher theoretical energy densities. On the solid electrolyte side, efforts are being made to develop a suitable material with improved ionic conductivity (∼ 10−3 S/cm) and enhanced electrochemical stability. SnF2-based solid electrolyte, BaSnF4 crystallizes in the PbSnF4 structure and is frequently considered as a potential solid electrolyte for FIBs operating at room temperature (RT). In this study, the conductivity results of BaSnF4 are influenced by doping with rare-earth ions (Ce3+) at different concentrations and by the synthesis methodology. The structural and transport behaviours of Ce3+-doped BaSnF4 solid electrolytes, prepared by mechanical milling and hydrothermal methods, are compared. The formation of the doped materials, which exhibit a tetragonal phase, is confirmed by X-ray diffraction. The presence of Ce3+ in the doped materials prepared by both methods is confirmed by their photoluminescence characteristics. Among the materials investigated in this study, 2 mol% Ce3+-doped BaSnF4, prepared by mechanical milling (Ba0.98Ce0.02SnF4.02-MM) exhibits the highest ionic conductivity and electrochemical stability. The conductivity (RT) exhibited by Ba0.98Ce0.02SnF4.02-MM is higher compared to earlier reports on different rare-earth ion-doped BaSnF4, which were primarily prepared by solution-based methods. Ion transport number measurement using dc polarization technique revealed that the conductivity exhibited by Ba0.98Ce0.02SnF4.02-MM is mainly due to ionic conduction. The observation of a higher ionic conductivity value in Ba0.98Ce0.02SnF4.02-MM highlights its great potential for use as a solid electrolyte in the fabrication of FIBs.
机械化学和水热合成Ce3+掺杂BaSnF4全固态氟离子电池固体电解质的结构和导电性研究
全固态氟离子电池(FIBs)具有较高的理论能量密度,是传统锂离子电池的潜在替代品,近年来的电池研究引起了人们的极大关注。在固体电解质方面,人们正在努力开发一种合适的材料,该材料具有改善的离子电导率(~ 10−3 S/cm)和增强的电化学稳定性。基于snf2的固体电解质,BaSnF4以PbSnF4结构结晶,经常被认为是在室温(RT)下工作的fib的潜在固体电解质。在本研究中,掺杂不同浓度的稀土离子(Ce3+)和合成方法对BaSnF4的电导率结果产生了影响。比较了机械研磨法和水热法制备的Ce3+掺杂BaSnF4固体电解质的结构和输运行为。x射线衍射证实了掺杂材料的形成,其表现为四方相。两种方法制备的掺杂材料的光致发光特性证实了Ce3+的存在。在所研究的材料中,机械铣削法制备的2 mol% Ce3+掺杂BaSnF4 (Ba0.98Ce0.02SnF4.02-MM)具有最高的离子电导率和电化学稳定性。与先前报道的不同稀土离子掺杂BaSnF4相比,Ba0.98Ce0.02SnF4.02-MM的电导率(RT)更高,主要是通过溶液法制备的。利用直流极化技术测量离子输运数,发现Ba0.98Ce0.02SnF4.02-MM的电导率主要是离子传导。在Ba0.98Ce0.02SnF4.02-MM中观察到较高的离子电导率值,突出了其作为固体电解质在FIBs制造中的巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Solid State Ionics
物理-物理:凝聚态物理
CiteScore
6.10
自引率
3.10%
发文量
152
审稿时长
58 days
期刊介绍:
This interdisciplinary journal is devoted to the physics, chemistry and materials science of diffusion, mass transport, and reactivity of solids. The major part of each issue is devoted to articles on:
(i) physics and chemistry of defects in solids;
(ii) reactions in and on solids, e.g. intercalation, corrosion, oxidation, sintering;
(iii) ion transport measurements, mechanisms and theory;
(iv) solid state electrochemistry;
(v) ionically-electronically mixed conducting solids.
Related technological applications are also included, provided their characteristics are interpreted in terms of the basic solid state properties.
Review papers and relevant symposium proceedings are welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


