Modification of platinum surfaces with cerium species for promoting oxidative desorption of adsorbed sulfur
IF 4.2
3区 工程技术
Q2 ELECTROCHEMISTRY
引用次数: 0
Abstract
Adsorption of sulfur (S) significantly reduces the electrochemically active surface area of platinum (Pt) electrocatalysts in polymer electrolyte membrane fuel cells (PEMFCs), namely, S poisoning. Mitigation techniques against S poisoning are strongly desired for highly durable PEMFCs. A Pt single-crystal surface was demonstrated to be modified with cerium (Ce) species by being immersed in a Ce-containing aqueous solution with hydrogen (H2) gas bubbling or potential holding at −0.2 V vs. Ag/AgCl. For a Ce-free Pt electrode, electrochemical responses characteristic of the adsorption/desorption of hydrogen and hydroxyl species at the bare Pt surface disappeared due to the adsorbed elemental sulfur, Sad, while the oxidative desorption of Sad from the Pt electrode occurred at around 0.80 V vs. Ag/AgCl. In contrast, for the Ce-modified Pt electrode, the oxidative desorption of Sad occurred at a potential around 0.3 V which is less positive (more negative) than that of Ce-free Pt electrode, showing the enhanced oxidative desorption capability due to the presence of Ce species on the surface. While the Ce species was desorbed from the Pt electrode simultaneously with the oxidative desorption of Sad, the Pt surface can be re-modified with the Ce species by H2 gas bubbling or potential holding at −0.2 V vs. Ag/AgCl, which is a similar condition to that of anode of PEMFC under operations. Thus, the Ce-modification of Pt surfaces potentially acts as a practical mitigation measure against the S poisoning.
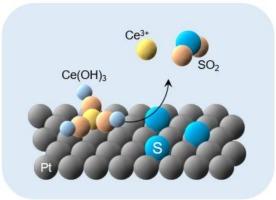
用铈修饰铂表面促进硫的氧化脱附
硫(S)的吸附显著降低了聚合物电解质膜燃料电池(pemfc)中铂(Pt)电催化剂的电化学活性表面积,即S中毒。对于高度耐用的pemfc,迫切需要针对S中毒的缓解技术。将Pt单晶表面浸泡在含Ce的水溶液中,使氢气(H2)鼓泡或电位保持在−0.2 V /Ag /AgCl下,证明了铈(Ce)可以修饰Pt单晶表面。对于无ce Pt电极,由于吸附了单质硫(Sad),裸Pt表面的氢和羟基的吸附/解吸特征消失,而在0.80 V /Ag /AgCl左右,Pt电极上的Sad发生氧化解吸。相比之下,对于Ce修饰的Pt电极,Sad的氧化脱附发生在0.3 V左右的电位下,该电位比无Ce修饰的Pt电极的正极电位少(负极电位多),表明由于表面存在Ce物质而增强了氧化脱附能力。Ce在Pt电极上的脱附过程与Sad的氧化脱附过程同时进行,通过H2气体鼓泡或电位保持在- 0.2 V vs. Ag/AgCl下,Ce可以在Pt表面进行再修饰,这与PEMFC阳极的操作条件相似。因此,铂表面的ce修饰有可能作为一种实际的缓解S中毒的措施。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochemistry Communications
工程技术-电化学
CiteScore
8.50
自引率
3.70%
发文量
160
审稿时长
1.2 months
期刊介绍:
Electrochemistry Communications is an open access journal providing fast dissemination of short communications, full communications and mini reviews covering the whole field of electrochemistry which merit urgent publication. Short communications are limited to a maximum of 20,000 characters (including spaces) while full communications and mini reviews are limited to 25,000 characters (including spaces). Supplementary information is permitted for full communications and mini reviews but not for short communications. We aim to be the fastest journal in electrochemistry for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


