Adsorption mechanism of H2O/oleate on α-quartz (101) surface with Al and Fe impurities/cations: DFT study and experimental verification
IF 1.8
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
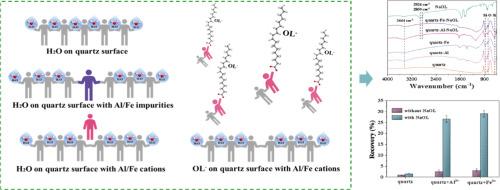
Flotation is an indispensable approach for efficiently separating gangue minerals from phosphate rock, with significant implications for the development and utilization of mineral resources in a highly efficient and sustainable manner. The Al and Fe elements are incorporated as impurity defects within quartz crystals, which alter the physicochemical properties of quartz minerals and subsequently influence their flotation behavior. The present study investigated the impact of Al and Fe impurity defects on the crystal structure, electronic characteristics, and surface wettability of α-quartz through density function theory (DFT). The calculation results suggest that the α-quartz with impurity defects alter the structure crystal and electronic structure properties of α-quartz. The presence of impurity defect further raises the adsorption energy of the H2O molecule and decreases the interaction between the α-quartz (101) surface and the H2O molecule. In addition, the wettability of α-quartz (101) hydrated surface treated with Al3+ and Fe3+ and its adsorption effect on the H2O molecule or oleate ion (OL-) were studied by DFT and experimental. The findings testify that OL- can be adsorbed on α-quartz (101) hydrated surface via Al3+ and Fe3+, thereby enhancing the hydrophobicity of quartz surface and improving natural flotation recovery. The computational prediction was validated by experimental results. Consequently, the existence of Al and Fe impurities/cations can improve the surface wettability of α-quartz, which is conducive to enhancing the natural floatability and provides valuable guidance for the flotation process.
Al和Fe杂质/阳离子在α-石英(101)表面的吸附机理:DFT研究和实验验证
浮选是有效分离磷矿石中脉石矿物的重要手段,对矿产资源的高效、可持续开发利用具有重要意义。Al和Fe元素作为杂质缺陷存在于石英晶体中,改变了石英矿物的物理化学性质,进而影响其浮选行为。利用密度泛函理论(DFT)研究了Al和Fe杂质缺陷对α-石英晶体结构、电子特性和表面润湿性的影响。计算结果表明,含有杂质缺陷的α-石英改变了α-石英的结构、晶体和电子结构性质。杂质缺陷的存在进一步提高了H2O分子的吸附能,降低了α-石英(101)表面与H2O分子的相互作用。此外,通过DFT和实验研究了Al3+和Fe3+处理α-石英(101)水合表面的润湿性及其对水分子或油酸离子(OL-)的吸附效果。结果表明,OL-可以通过Al3+和Fe3+吸附在α-石英(101)水化表面,从而增强石英表面的疏水性,提高自然浮选回收率。实验结果验证了计算预测的正确性。因此,Al和Fe杂质/阳离子的存在可以改善α-石英的表面润湿性,有利于提高天然可浮性,对浮选过程具有重要的指导意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


