Microbial degradation behavior and mechanism of polyamide 6 fibers in seawater
IF 7.4
2区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
Polyamide 6 (PA6) is a widely used thermoplastic polymer. Compared to other nylons, it offers good mechanical properties as well as chemical and thermal stability. Difficult to degrade and coupled with its massive annual global production, it has rapidly become a significant ecological pollutant. Therefore, developing environmentally friendly degradation methods is an urgent need, among which microbial degradation represents a promising solution. This study conducted laboratory-simulated seawater degradation experiments on polyamide 6 (PA6) fibers. The structural changes of PA6 over a 190-day degradation period were analyzed using characterization techniques, including scanning electron microscopy, relative viscosity, and differential scanning calorimetry. Results demonstrated significant and irregular degradation of PA6. Prolonged degradation led to decreases in relative viscosity, number-average molecular weight, melting temperature, and melting enthalpy. FTIR and XPS analyses revealed cleavage of C–C, C–N, and C = O bonds, accompanied by intensified signals of C–OH and O = C–O–C groups, indicating hydrolysis of amide bonds to generate terminal amino and carboxyl groups. Degradation product analysis suggested that the initial step of PA6 degradation primarily produced tetrameric and cyclic dimeric forms of 6-aminohexanoic acid. Further microbial community analysis and functional annotation identified Proteobacteria as the dominant microbial population responsible for PA6 degradation. This study provides new insights into the degradation behavior of polyamide fibers in marine environments and contributes substantially to solving the problem of marine polyamide waste.
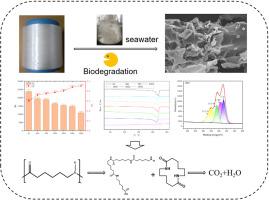
聚酰胺6纤维在海水中的微生物降解行为及机理
聚酰胺6 (PA6)是一种应用广泛的热塑性聚合物。与其他尼龙相比,它具有良好的机械性能以及化学和热稳定性。由于其难以降解,再加上其每年在全球范围内的大量产量,它已迅速成为一种重要的生态污染物。因此,开发环境友好的降解方法是迫切需要的,其中微生物降解是一个很有前途的解决方案。本研究对聚酰胺6 (PA6)纤维进行了实验室模拟海水降解实验。利用扫描电镜、相对粘度、差示扫描量热等表征技术分析了PA6在190天降解过程中的结构变化。结果表明PA6的降解明显且不规则。长时间降解导致相对粘度、数平均分子量、熔化温度和熔化焓降低。FTIR和XPS分析显示,C - C、C - n和C = O键发生断裂,同时C - oh和O = C - O - C基团信号增强,表明酰胺键水解生成末端氨基和羧基。降解产物分析表明,PA6降解的初始阶段主要产生四聚体和环二聚体形式的6-氨基己酸。进一步的微生物群落分析和功能注释确定Proteobacteria是主要的降解PA6的微生物群体。本研究对聚酰胺纤维在海洋环境中的降解行为提供了新的认识,对解决海洋聚酰胺废弃物问题具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Degradation and Stability
化学-高分子科学
CiteScore
10.10
自引率
10.20%
发文量
325
审稿时长
23 days
期刊介绍:
Polymer Degradation and Stability deals with the degradation reactions and their control which are a major preoccupation of practitioners of the many and diverse aspects of modern polymer technology.
Deteriorative reactions occur during processing, when polymers are subjected to heat, oxygen and mechanical stress, and during the useful life of the materials when oxygen and sunlight are the most important degradative agencies. In more specialised applications, degradation may be induced by high energy radiation, ozone, atmospheric pollutants, mechanical stress, biological action, hydrolysis and many other influences. The mechanisms of these reactions and stabilisation processes must be understood if the technology and application of polymers are to continue to advance. The reporting of investigations of this kind is therefore a major function of this journal.
However there are also new developments in polymer technology in which degradation processes find positive applications. For example, photodegradable plastics are now available, the recycling of polymeric products will become increasingly important, degradation and combustion studies are involved in the definition of the fire hazards which are associated with polymeric materials and the microelectronics industry is vitally dependent upon polymer degradation in the manufacture of its circuitry. Polymer properties may also be improved by processes like curing and grafting, the chemistry of which can be closely related to that which causes physical deterioration in other circumstances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


