Tricoordinate copper(I) complexes of N,N-bidentate Schiff-base ligand: Syntheses, crystal structure determinations, electrochemical properties, theoretical studies, and catalytic activities
IF 5.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
This study delves into the synthesis and characterization of tricoordinate Cu(I) Schiff-base complexes derived from N,N′-bis(4-chlorobenzylidene)-2,2-dimethylpropane-1,3-diamine, focusing on their catalytic applications. The investigation explores the spectroscopic features, crystal structures, and theoretical properties of these complexes, shedding light on their structural diversity and catalytic potential. Through DFT calculations, NBO analyses, and QTAIM studies, the intricate intermolecular interactions and electronic properties of the Cu(I) complexes are elucidated. Additionally, the redox behavior of these complexes at the glassy carbon electrode is explored, revealing quasi-reversible redox processes. The catalytic activity of the Cu(I) complexes in synthesizing heterocyclic compounds is evaluated, showcasing their effectiveness in promoting target product formation under mild conditions. This comprehensive study underscores the significance of Cu(I) Schiff-base complexes in catalysis and highlights their potential for various applications in organic synthesis.
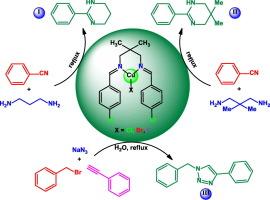
N,N双齿希夫碱配体的三配位铜(I)配合物:合成、晶体结构测定、电化学性质、理论研究和催化活性
本研究研究了N,N ' -双(4-氯苄二胺)-2,2-二甲基丙烷-1,3-二胺衍生的三配位Cu(I)希夫碱配合物的合成和表征,重点研究了它们的催化应用。研究探讨了这些配合物的光谱特征、晶体结构和理论性质,揭示了它们的结构多样性和催化潜力。通过DFT计算、NBO分析和QTAIM研究,阐明了Cu(I)配合物复杂的分子间相互作用和电子性质。此外,研究了这些配合物在玻碳电极上的氧化还原行为,揭示了准可逆的氧化还原过程。对Cu(I)配合物在杂环化合物合成中的催化活性进行了评价,显示了其在温和条件下促进目标产物生成的有效性。这项综合研究强调了Cu(I)席夫碱配合物在催化中的重要性,并强调了它们在有机合成中的各种应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


