Factor X and combined factor VIIa/factor X augment coagulation potential in a plasma model of antithrombin-reduced hemophilia
IF 3.4
3区 医学
Q2 HEMATOLOGY
Research and Practice in Thrombosis and Haemostasis
Pub Date : 2025-08-01
DOI:10.1016/j.rpth.2025.103172
引用次数: 0
Abstract
Background
Plasma-derived (pd) factor (F)VIIa/FX products are available for the hemostatic management of people with hemophilia with inhibitors in Japan. We have previously reported that FX alone augments emicizumab-driven hemostasis. Fitusiran is an investigational small interfering RNA that reduces antithrombin (AT) to rebalance hemostasis in people with hemophilia. The effects of supplementation with pd-FVIIa/FX or FX alone on coagulation potential in AT-reduced hemophilia remain to be clarified.
Objectives
To assess the coagulation potential of pd-FVIIa/FX or FX using an in vitro model of AT-reduced (fitusiran-treated) people with hemophilia.
Methods
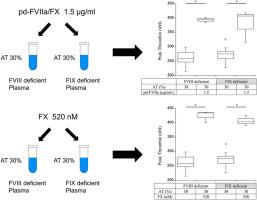
Pd-FVIIa/FX (0.75 and 1.5 μg/mL as FVIIa), recombinant FVIIa (1.1 and 2.2 μg/mL), activated prothrombin complex concentrate (0.65 and 1.3 IU/mL), or FX (260 and 520 nM) were added to FVIII- or FIX-depleted AT-deficient plasmas at AT levels of 10% and 30% (AT-reduced model). Additionally, FX (0-1040 nM) was incubated with FVIII- or FIX-depleted FX-deficient plasmas to assess FVIIa/tissue factor (TF)-mediated FX activation. Coagulation potential was assessed by measuring thrombin generation (TG) and FXa.
Results
The addition of pd-FVIIa/FX, activated prothrombin complex concentrate, or FX to the AT-reduced plasma model of people with hemophilia improved TG potential within the normal range. The addition of recombinant FVIIa, however, had little effect on TG in this model. The addition of FX to FVIII- or FIX-depleted FX-deficient plasma increased TG potential and FVIIa/TF-triggered FXa generation dose-dependently.
Conclusion
Supplementation with FX or pd-FVIIa/FX enhanced FVIIa/TF-induced activation of FX and increased coagulation potential in the AT-reduced plasma model of people with hemophilia.
因子X和联合因子VIIa/因子X增强抗凝血酶降低血友病血浆模型中的凝血电位
背景:血浆源性(pd)因子(F)VIIa/FX产品可用于血友病患者的抑制剂止血管理。我们之前报道过单独使用FX可以增强半单抗驱动的止血作用。Fitusiran是一种实验性小干扰RNA,可降低抗凝血酶(AT)以重新平衡血友病患者的止血。补充pd-FVIIa/FX或单独使用FX对at减少血友病患者凝血电位的影响仍有待澄清。目的利用at减少(菲图西兰治疗)血友病患者的体外模型,评估pd-FVIIa/FX或FX的凝血潜能。方法将pd -FVIIa/FX(作为FVIIa的0.75和1.5 μg/mL)、重组FVIIa(1.1和2.2 μg/mL)、活化凝血酶原复合物浓缩物(0.65和1.3 IU/mL)或FX(260和520 nM)分别以at水平10%和30% (at减少模型)添加到FVIII或fix -贫at缺陷血浆中。此外,FX (0-1040 nM)与FVIII-或fix -耗尽的FX-缺陷血浆孵育,以评估FVIIa/组织因子(TF)介导的FX激活。通过测定凝血酶生成(TG)和FXa来评估凝血电位。结果血友病患者at还原血浆模型中加入pd-FVIIa/FX、活化凝血酶原复合物浓缩物或FX后,TG电位在正常范围内得到改善。然而,在该模型中,重组FVIIa的加入对TG的影响很小。在FVIII-或fix -耗尽的FX-缺陷血浆中添加FX可增加TG电位和FVIIa/ tf触发的FXa生成剂量依赖性。结论补充FX或pd-FVIIa/FX可增强血友病患者at减少血浆模型中FVIIa/ tf诱导的FX活化和凝血电位升高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Research and Practice in Thrombosis and Haemostasis
Medicine-Hematology
CiteScore
5.60
自引率
13.00%
发文量
212
审稿时长
7 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


