Photophysical properties of 5-methoxyflavone and its protonated species influenced by intermolecular hydrogen bonding and environmental rigidity
IF 4.2
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The excited-state intramolecular proton transfer (ESPTintra) process in flavonoids has attracted considerable attention due to its fundamental role in their photophysics. This study systematically investigates the photophysical properties of 5-methoxyflavone (5MF), a flavonoid derivative incapable of ESPTintra. It was demonstrated that intermolecular hydrogen bonding with protic solvents effectively suppress the non-radiative ππ∗-nπ∗ internal conversion in 5MF, leading to significant fluorescence enhancement. In highly acidic aqueous solutions (pH < 3.3), an excited-state intermolecular proton transfer (ESPTinter) process takes place, generating protonated 5MF (5MFH+) and resulting in fluorescence quenching. Notably, when incorporated in acidic poly(vinyl alcohol) (PVA) films, the excited-state protonated species 5MFH+ exhibits intense fluorescence with a long fluorescence lifetime of 8.4 ns, owing to the suppression of non-radiative decay pathways by the rigid polymer network. Additionally, the dense hydroxyl groups in the PVA matrix also enhance the emission of neutral 5MF. These results highlight the potential of both 5MF and 5MFH+ as efficient luminescent chromophores for the design of optical materials based on hydrogen-bonding polymer systems.
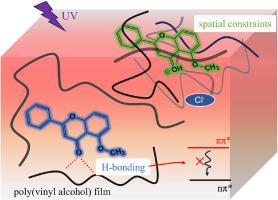
5-甲氧基黄酮及其质子化物质的光物理性质受分子间氢键和环境刚性的影响
黄酮类化合物的激发态分子内质子转移(ESPTintra)过程因其在黄酮类化合物光物理中的基础性作用而受到广泛关注。本研究系统地研究了5-甲氧基黄酮(5-methoxyflavone, 5MF)的光物理性质。结果表明,与质子溶剂的分子间氢键有效抑制了5MF中非辐射ππ∗-nπ∗的内部转换,导致显著的荧光增强。在高酸性水溶液(pH < 3.3)中,发生激发态分子间质子转移(ESPTinter)过程,产生质子化5MF (5MFH+),导致荧光猝灭。值得注意的是,当加入酸性聚乙烯醇(PVA)薄膜时,激发态质子化物质5MFH+表现出强烈的荧光,荧光寿命长达8.4 ns,这是由于刚性聚合物网络抑制了非辐射衰变途径。此外,PVA基质中密集的羟基也增强了中性5MF的发射。这些结果突出了5MF和5MFH+作为基于氢键聚合物体系的光学材料设计的高效发光发色团的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


