Visible-Light-Induced Regioselective Benzylic CH Oxidation of Substituted 6-Aryl-7,8,9,10-tetrahydrobenzo[c]phenanthridine
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
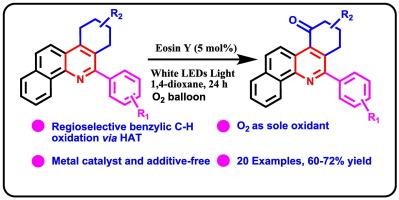
A practical and sustainable protocol for the photocatalytic oxidation of benzylic C![]() H bonds, particularly within polycyclic heteroaromatic (PHA) frameworks, has been developed. In contrast to many previously reported systems, which often rely on long reaction times, narrow-spectrum LEDs, additives, or specialized photoreactors or setups, this method employs inexpensive eosin Y under white LED illumination with molecular oxygen supplied via a balloon. The transformation proceeds under mild conditions without the need for transition metals, hazardous oxidants, or auxiliary additives, and operates via a radical mechanism supported by inhibition studies. The broad substrate tolerance and operational simplicity of this protocol facilitate efficient access to benzylic ketones. Its utility is exemplified in the synthesis of a variety of 6-aryl-8,9-dihydrobenzo[c]phenanthridin-10(7H)-one derivatives, where the substituent at the 6-position plays a critical role in determining product outcome. This method represents one of the few reported examples of late-stage C
H bonds, particularly within polycyclic heteroaromatic (PHA) frameworks, has been developed. In contrast to many previously reported systems, which often rely on long reaction times, narrow-spectrum LEDs, additives, or specialized photoreactors or setups, this method employs inexpensive eosin Y under white LED illumination with molecular oxygen supplied via a balloon. The transformation proceeds under mild conditions without the need for transition metals, hazardous oxidants, or auxiliary additives, and operates via a radical mechanism supported by inhibition studies. The broad substrate tolerance and operational simplicity of this protocol facilitate efficient access to benzylic ketones. Its utility is exemplified in the synthesis of a variety of 6-aryl-8,9-dihydrobenzo[c]phenanthridin-10(7H)-one derivatives, where the substituent at the 6-position plays a critical role in determining product outcome. This method represents one of the few reported examples of late-stage C![]() H functionalization in PHAs under photocatalytic conditions.
H functionalization in PHAs under photocatalytic conditions.
可见光诱导取代6-芳基-7,8,9,10-四氢苯并[c]菲蒽啶的区域选择性苄基CH氧化
一个实用的和可持续的方案,光催化氧化的苄基CH键,特别是在多环杂芳烃(PHA)框架,已经开发。与许多先前报道的系统相比,这些系统通常依赖于长反应时间、窄光谱LED、添加剂或专门的光反应器或装置,该方法在白光LED照明下使用廉价的伊红Y,并通过气球提供分子氧。这种转化在温和的条件下进行,不需要过渡金属、有害氧化剂或辅助添加剂,并通过抑制研究支持的自由基机制进行。该方法广泛的底物耐受性和操作简单性促进了对苯酮的有效获取。它的用途在合成各种6-芳基-8,9-二氢苯并[c]菲蒽啶-10(7H)- 1衍生物中得到了例证,其中6-位置的取代基在决定产物产物中起着关键作用。该方法是少有报道的光催化条件下相芳烃中晚期CH功能化的例子之一。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


