Advancement in electrochemical sensing of chloramphenicol in varying matrixes: A review
IF 3.7
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
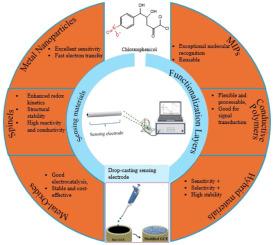
Chloramphenicol (CAP) have demonstrated its broad effectiveness against bacterial infections. However, its persistence in the environment and potential toxicity led to strict global regulations limiting its use. As a result, rapid and accurate detection methods for CAP are being developed to protect public health and maintain regulatory compliance regarding its presence in the environment. Conventional analytical methods such as High-Performance Liquid Chromatography, Gas Chromatography, and Liquid Chromatography–Mass Spectrometry were commonly used for CAP detection. However, these conventional methods suffer challenges such as time-consumption, fabrication complexity, reproducibility, and cost. With electrochemical sensing techniques presenting varying valuable benefits, such as instant detection, low power consumption, simultaneous analysis, and portability, making them essential for timely monitoring of various analytes. The performance of electrochemical sensor is further boosted by integrating materials such as spinels, metal oxides, and metallic nanoparticles. These materials are broadly investigated as electrode interface components thanks to their features that collectively boost electrochemical sensor such as providing excellent electrical conductivity, distinct catalytic behavior, and larger surface areas. Furthermore, this paper reviews the impact of traditional and standards analytical methods, as well as their recent advancement based on the detection of CAP in aqueous media.
氯霉素在不同基质中的电化学传感研究进展
氯霉素(CAP)已被证明对细菌感染具有广泛的有效性。然而,它在环境中的持久性和潜在的毒性导致严格的全球法规限制其使用。因此,正在开发快速和准确的CAP检测方法,以保护公众健康并保持其在环境中存在的法规遵从性。高效液相色谱法、气相色谱法、液相色谱-质谱法等常规分析方法是CAP检测常用的方法。然而,这些传统方法面临着诸如耗时、制造复杂性、可重复性和成本等挑战。随着电化学传感技术呈现出各种有价值的优点,如即时检测,低功耗,同时分析和便携性,使其成为及时监测各种分析物的必要条件。尖晶石、金属氧化物、金属纳米颗粒等材料的集成进一步提高了电化学传感器的性能。这些材料作为电极界面组件被广泛研究,这要归功于它们共同提升电化学传感器的特性,如提供优异的导电性、独特的催化行为和更大的表面积。此外,本文综述了传统分析方法和标准分析方法的影响,以及基于水介质中CAP检测的最新进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


