Revolutionizing antibiotic elimination: g-C3N4 modified Al2Cr2O6 nanocomposite for outstanding adsorption of ciprofloxacin
IF 5.1
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
Abstract
Artificial organic compounds, including antibiotics, represent substantial organic pollutants in wastewater; their remediation is essential. A new g-C3N4 modified Al2Cr2O6 nanocomposite serves as an effective platform for the elimination of Ciprofloxacin (Cipro), an antibiotic pollutant. This study developed and evaluated the g-C3N4@Al2Cr2O6 nanocomposite as a sorbent to enhance the treatment of pharmaceutical wastewater containing Cipro. Analytical instruments, including XRD, EDX, XPS, BET, SEM, and TEM, were employed to characterize the synthesized adsorbent. The adsorption ability of the g-C3N4@Al2Cr2O6 nanocomposite for Cipro was investigated using a batch experimental method under various conditions. The results demonstrated that the maximum adsorption capacity of Cipro was attained after 35 min of contact time at optimal conditions of pH 7 and a temperature of 25 °C. Analysis of thermodynamic parameters reveals that both ΔG° and ΔH° are negative. As a result, adsorption takes place spontaneously and displays an exothermic nature. The Sips model indicates that the g-C3N4@Al2Cr2O6 nanocomposite had an adsorption capacity of 95.99 mg/g. The adsorptive uptake kinetics demonstrated adherence to pseudo-second-order (PSO) kinetics. The g-C3N4@Al2Cr2O6 nanocomposite demonstrated a higher adsorption efficiency and exhibited favorable structural stability, as evidenced by XRD. The principal Cipro modes of adsorption were hydrogen bonding, hydrophobic interactions, electrostatic attraction, complexation, and π-π interactions. Our research demonstrates that the adsorbents are a stable, efficient, and recyclable nanocomposite for Cipro adsorption.
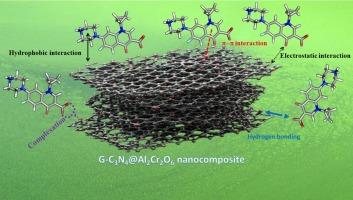
革命性的抗生素消除:g-C3N4修饰的Al2Cr2O6纳米复合材料具有出色的环丙沙星吸附
人工有机化合物,包括抗生素,是废水中大量的有机污染物;他们的补救是必要的。一种新的g-C3N4修饰的Al2Cr2O6纳米复合材料为消除抗生素污染物环丙沙星(Cipro)提供了有效的平台。本研究开发并评估了g-C3N4@Al2Cr2O6纳米复合材料作为吸附剂对含环丙沙星的制药废水的处理效果。采用XRD、EDX、XPS、BET、SEM、TEM等分析仪器对合成的吸附剂进行了表征。采用间歇式实验方法研究了g-C3N4@Al2Cr2O6纳米复合材料在不同条件下对环丙沙星的吸附能力。结果表明,在pH为7、温度为25℃的最佳条件下,接触时间为35 min时,环丙酚的吸附量达到最大。热力学参数分析表明ΔG°和ΔH°均为负值。因此,吸附是自发发生的,并表现为放热性质。Sips模型表明g-C3N4@Al2Cr2O6纳米复合材料的吸附量为95.99 mg/g。吸附吸收动力学表现出伪二阶动力学。XRD结果表明,g-C3N4@Al2Cr2O6纳米复合材料具有较高的吸附效率和良好的结构稳定性。Cipro吸附的主要模式是氢键、疏水相互作用、静电吸引、络合和π-π相互作用。我们的研究表明,该吸附剂是一种稳定、高效、可回收的环丙沙星吸附纳米复合材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


