Bisphenol S drives breast cancer metastasis by inhibiting dopamine receptor D2 to activate the Akt/GSK3β oncogenic axis
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Bisphenol S (BPS), a substitute for bisphenol A (BPA), serves as an endocrine disruptor implicated in breast cancer (BC) progression, but its mechanisms remain unclear.Objectives
This study aimed to elucidate the underlying mechanisms of BPS effects on BC metastasis through in vivo and in vitro experiments.Methods
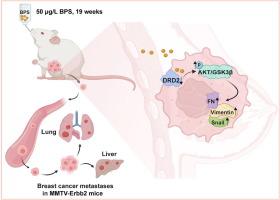
Transgenic MMTV-Erbb2 mice that spontaneously developed breast tumors were orally administered BPS (50 μg/L) for 19 weeks, and BC cells were exposed to BPS (0, 0.01, 0.1, or 1 μM) for 72 h to examine the effects of BPS exposure on the migration and invasion of BC cells. Furthermore, we employed transcriptomics, KEGG enrichment analysis and Comparative Toxicogenomics Database (CTD) predictions to explore the potential mechanisms underlying BPS −induced BC metastasis. In addition, we used tissue microarray (TMA) and public databases to reveal DRD2′s critical role in BC progression.Results
Our results indicated that low-dose BPS (50 μg/L, approximately 6.14 μg/kg/day) facilitated BC metastasis both in vitro and in vivo. Transcriptomic analysis identified DRD2 as a critical regulator of migration and invasion in BPS-exposed MCF-7 cells. Furthermore, KEGG enrichment analysis coupled with the CTD demonstrated that BPS enhanced the migration and invasion of BC cells via the Akt/GSK3β signaling pathway activation. Importantly, DRD2 overexpression apparently blocked the Akt/GSK3β pathway, effectively reversing BPS-induced metastasis of BC both in vitro and in vivo. Intriguingly, TMA and public database analyses revealed a marked decrease of DRD2 levels in BC tissues, which were inversely correlated with p-Akt levels in BC tissues and positively associated with the poor clinical characteristics of BC patients.Conclusion
BPS exposure reduces DRD2 levels, activating Akt/GSK3β signaling to drive BC metastasis. These findings highlight the risk of BPS as a BPA alternative and unravel mechanistic insights into the role of environmental endocrine disruptors in cancer progression.
双酚S通过抑制多巴胺受体D2激活Akt/GSK3β致癌轴来驱动乳腺癌转移
双酚S (BPS)是双酚a (BPA)的替代品,作为内分泌干扰物与乳腺癌(BC)进展有关,但其机制尚不清楚。目的通过体内和体外实验,探讨BPS对乳腺癌转移的影响机制。方法对自发发生乳腺肿瘤的转基因MMTV-Erbb2小鼠口服BPS(50 μg/L) 19 周,将BC细胞暴露于BPS(0、0.01、0.1或1 μM)中72 h,观察BPS暴露对BC细胞迁移和侵袭的影响。此外,我们利用转录组学、KEGG富集分析和比较毒物基因组学数据库(CTD)预测来探索BPS−诱导的BC转移的潜在机制。此外,我们使用组织微阵列(TMA)和公共数据库来揭示DRD2在BC进展中的关键作用。结果低剂量BPS(50 μg/L,约6.14 μg/kg/天)促进BC在体外和体内转移。转录组学分析发现DRD2是bps暴露的MCF-7细胞迁移和侵袭的关键调节因子。此外,KEGG富集分析与CTD结合表明,BPS通过激活Akt/GSK3β信号通路增强了BC细胞的迁移和侵袭。重要的是,DRD2过表达明显阻断了Akt/GSK3β通路,在体外和体内均有效逆转了bps诱导的BC转移。有趣的是,TMA和公共数据库分析显示,BC组织中DRD2水平显著降低,这与BC组织中p-Akt水平呈负相关,与BC患者的不良临床特征呈正相关。结论bps暴露可降低DRD2水平,激活Akt/GSK3β信号通路,促进BC转移。这些发现强调了BPS作为双酚a替代品的风险,并揭示了环境内分泌干扰物在癌症进展中的作用机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


