Hydroxyl anchoring and electron transfer behaviors of atomic single-layer Pt in NH3 oxidation
IF 11.6
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
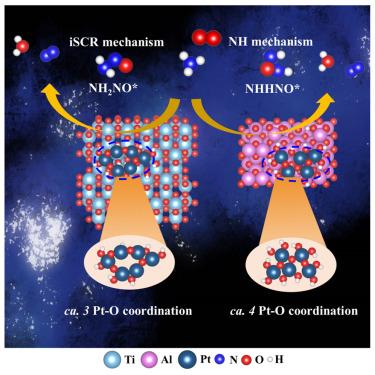
Platinum (Pt) atomic single-layer (ASL) can trigger synergistic effects between metal atoms and surface moieties of supports, which governs its catalytic activity and product selectivity. The electronic metal-support interaction determines not only the local coordination environment in shaping the stability and reactivity of Pt on support, but also the covalency of Pt–O and electron transfer properties. Here, we reveal the impact of different anchoring mechanisms between Pt ASL and supports on catalytic activity and product selectivity of NH3 oxidation. The Pt ASL consumes more low-coordination terminal hydroxyls on Al2O3, and the lower coordination number leads to the stronger electron transfer between Pt ASL and TiO2. The imino group acts as the key intermediate on Pt/TiO2 resulting in higher NH3 conversion but lower N2 selectivity, whereas the amino group plays a dominant role on Pt/Al2O3 leading to slightly lower NH3 conversion but higher N2 selectivity because of rapid NH3∗ dehydrogenation.
原子单层Pt在NH3氧化中的羟基锚定和电子转移行为
铂(Pt)原子单层(ASL)可以触发金属原子与载体表面基团之间的协同作用,从而控制其催化活性和产物选择性。电子金属-载体相互作用不仅决定了Pt在载体上形成稳定性和反应性的局部配位环境,而且决定了Pt - o的共价和电子转移性质。在这里,我们揭示了Pt ASL和载体之间不同的锚定机制对NH3氧化的催化活性和产物选择性的影响。Pt ASL在Al2O3上消耗的低配位末端羟基较多,配位数越低,Pt ASL与TiO2之间的电子转移越强。在Pt/TiO2上,亚胺基作为关键中间体,NH3转化率较高,但N2选择性较低;而在Pt/Al2O3上,氨基起主导作用,NH3转化率略低,但N2选择性较高,因为NH3 *脱氢迅速。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


