Elementary steps and bifunctional scavenging pathways in methylcyclohexane dehydrogenation reactions on dispersed Pt nanoparticles
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Dehydrogenation-hydrogenation cycles of methylcyclohexane (MCH) and toluene (TOL) provide a feasible strategy for hydrogen release and storage. This study describes the kinetic relevance of MCH dehydrogenation on Pt and addresses the support effect on turnover rates, which involves kinetically-competent Lewis acid-base (LAB) pairs on the supports (e.g., Al2O3, TiO2, and ZrO2) that affect the bound species on Pt surfaces with varying structures and reactivities. The less competent species in producing TOL on Pt surfaces are present at significant coverages and can desorb and are scavenged by LAB sites on the support, thus increasing the number and size of Pt surface atom ensembles for the more competent ones to form TOL. The support effect on MCH dehydrogenation turnover rates diminishes upon propionic acid titration, which occupies LAB sites irreversibly. H2-D2 exchange and CO titration experiments under simultaneous MCH dehydrogenation confirm the significant coverage of the less competent species derived from MCH on Pt surfaces. For all investigated Pt catalysts, MCH dehydrogenation turnover rates depend linearly on pressures of methylcyclohexene (MCHE) as detectable intermediates that are in equilibrium with MCH and H2, indicating that the kinetically-relevant step occurs after MCHE formation and involves C–H activation in bound MCHE-derived species on Pt surfaces that are sparsely covered by reactive intermediates. The implications and practical significance of this work are not restricted to the subject reaction, and can be extended to other bifunctional catalytic systems.
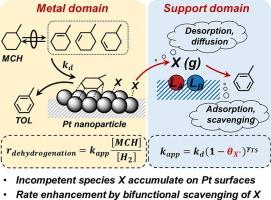
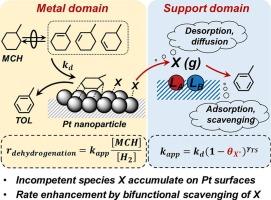
分散Pt纳米颗粒上甲基环己烷脱氢反应的基本步骤和双功能清除途径
甲基环己烷(MCH)和甲苯(TOL)的脱氢-加氢循环为氢的释放和储存提供了可行的策略。本研究描述了MCH在Pt上脱氢的动力学相关性,并解决了载体对周转率的影响,这涉及到载体(如Al2O3, TiO2和ZrO2)上的动力学能力Lewis酸碱(LAB)对,这些对会影响具有不同结构和反应活性的Pt表面上的结合物质。在Pt表面上产生TOL的能力较弱的物质在很大程度上存在,并且可以解吸并被载体上的LAB位点清除,从而增加了Pt表面原子群的数量和大小,为更有能力形成TOL的物质提供了条件。丙酸滴定后,载体对MCH脱氢周转率的影响减弱,丙酸不可逆地占据了LAB位点。同时MCH脱氢条件下的H2-D2交换和CO滴定实验证实了Pt表面上由MCH衍生的低活性物质的显著覆盖。对于所有被研究的Pt催化剂,MCH脱氢周转率线性依赖于甲基环己烯(MCHE)的压力,作为与MCH和H2平衡的可检测中间体,这表明动力学相关的步骤发生在MCHE形成之后,并且涉及在被反应中间体稀疏覆盖的Pt表面上结合的MCHE衍生物质的C-H活化。这项工作的意义和实际意义不仅限于主题反应,而且可以扩展到其他双功能催化体系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


