Orthosteric–allosteric dual inhibitors of PI5P4Kγ with potent antitumor activity in non-small cell lung cancer
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Phosphatidylinositol 5-phosphate 4-kinase type II gamma (PI5P4Kγ) has emerged as a promising therapeutic target in oncology due to its key role in cancer progression. However, currently available inhibitors targeting either the orthosteric or allosteric sites of PI5P4Kγ suffer from limited in vitro activity and in vivo validation.Objectives
This study aimed to develop a highly potent and selective PI5P4Kγ inhibitor capable of simultaneously engaging both the orthosteric and allosteric sites, as well as to validate their potential in therapy for non-small cell lung cancer (NSCLC) were investigated.Methods
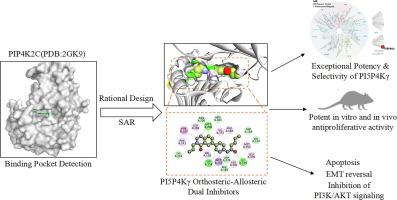
Through the structural modification of the propionamide side chain of the pyridine ring and the N3 substituent of the quinazolinone, the structure–activity relationship (SAR) was elucidated, leading to the identification of the target compound n40. The binding mode of n40 to PI5P4Kγ, was elucidated by X-ray crystallography. In vitro effects were assessed through KINOMEscan Technology, cell proliferation, apoptosis, and EMT analysis. In vivo efficacy was evaluated using an HCC827 xenograft mouse model. Both single-dose (up to 400 mg/kg) and repeated-dose (20–180 mg/kg/day for 28 days) toxicity studies of n40 were conducted in C57BL/6 mice, with comprehensive monitoring of physiological parameters and organ toxicity indices.Results
Compound n40 exhibited a strong binding affinity for PI5P4Kγ (Kd = 6.55 nM), with 3,000-fold selectivity over other two isoforms. Additionally, it displayed sub-50 nM antiproliferative activity in NSCLC cells with higher PI5P4Kγ levels. X-ray crystallography revealed n40 simultaneously binds to both orthosteric and a novel allosteric pocket. Functionally, n40 induced S-phase arrest, apoptosis, and EMT reversal and PI3K/AKT signaling inhibition in a PI5P4Kγ-dependent manner. In HCC827 xenografts, n40 achieved > 60 % tumor growth inhibition without any observed toxicity.Conclusion
This study establishes PI5P4Kγ as a druggable target in NSCLC and demonstrates that dual orthosteric–allosteric targeting is a viable strategy to achieve potent and selective PI5P4Kγ inhibitors.
在非小细胞肺癌中具有有效抗肿瘤活性的PI5P4Kγ正构-变构双抑制剂
磷脂酰肌醇5-磷酸4激酶II型γ (PI5P4Kγ)由于其在癌症进展中的关键作用而成为肿瘤治疗中有希望的治疗靶点。然而,目前可用的靶向PI5P4Kγ正构位点或变构位点的抑制剂体外活性和体内验证有限。本研究旨在开发一种高效、选择性的PI5P4Kγ抑制剂,能够同时作用于正构和变构位点,并验证其在非小细胞肺癌(NSCLC)治疗中的潜力。方法通过对吡啶环丙酰胺侧链和喹唑啉酮的N3取代基进行结构修饰,确定其构效关系(SAR),从而鉴定目标化合物n40。用x射线晶体学分析了n40与PI5P4Kγ的结合模式。通过KINOMEscan技术、细胞增殖、细胞凋亡和EMT分析来评估体外效果。使用HCC827异种移植小鼠模型评估体内疗效。对C57BL/6小鼠进行了n40单次给药(最多400 mg/kg)和重复给药(20-180 mg/kg/天,共28 天)的毒性研究,并对生理参数和器官毒性指标进行了全面监测。结果化合物n40对PI5P4Kγ具有较强的结合亲和力(Kd = 6.55 nM),选择性是其他两种亚型的3000倍。此外,它在PI5P4Kγ水平较高的NSCLC细胞中显示出低于50 nM的抗增殖活性。x射线晶体学显示n40同时与正构和一个新的变构口袋结合。在功能上,n40以pi5p4k - γ依赖的方式诱导s期阻滞、凋亡、EMT逆转和PI3K/AKT信号传导抑制。在HCC827异种移植物中,n40达到 >; 60 %的肿瘤生长抑制,没有观察到任何毒性。结论本研究确定PI5P4Kγ是NSCLC的可药物靶点,并证明双正构-变构靶向是获得有效和选择性PI5P4Kγ抑制剂的可行策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


