Development and clinical validation of a novel protein biomarkers-based algorithm for risk prediction and diagnosis of advanced liver fibrosis: a multi-centre study
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Type 2 diabetes (T2D) and obesity contribute significantly to the elevated risk of liver fibrosis in metabolic dysfunction-associated steatotic liver disease (MASLD). However, there is a lack of reliable and cost-effective non-invasive test (NIT) for detecting liver fibrosis in T2D/obese individuals.Objectives
This study aimed to develop a simple biomarker-based algorithm for detecting advanced liver fibrosis among T2D/obesity subjects with MASLD and to validate its diagnostic performance in both clinic- and community-based cohorts.Methods
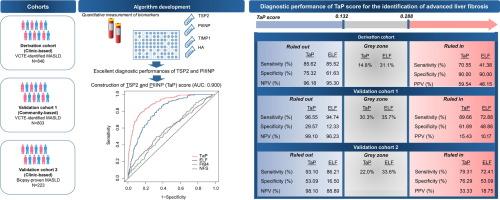
Diagnostic performances of circulating thrombospondin-2 (TSP2), a novel fibrosis marker, and the three individual components of Enhanced Liver Fibrosis (ELF) test were evaluated in three independent cohorts. These included a clinic-based derivation cohort (N = 846) and a community-based validation cohort (N = 803), both comprising of T2D patients with vibration-controlled transient elastography (VCTE)-diagnosed MASLD. Additionally, a clinic-based validation cohort of morbidly-obese patients with biopsy-proven MASLD (N = 223) was included. An algorithm (TaP score) based on TSP2 and procollagen 3 N-terminal peptide (PIIINP), a component of ELF, was constructed from the multivariate logistic regression model and compared with existing NITs, including ELF, fibrosis-4 index (FIB-4) and NAFLD fibrosis score (NFS). The dual-cut-off approach was used to define the rule-in and rule-out cut-offs.Results
Circulating TSP2 (AUC[95 %CI]:0.844[0.810–0.878]) and PIIINP (AUC[95 %CI]:0.843(0.807–0.875]) showed excellent diagnostic performance and were used to construct the biomarker-based algorithm. The TaP score (AUC[95 %CI]:0.900[0.874–0.925]) significantly outperformed ELF (AUC[95 %CI]:0.809[0.773–0.843]), FIB-4 (AUC[95 %CI]:0.597[0.544–0.647]) and NFS (AUC[95 %CI]:0.585[0.528–0.639]) (all DeLong P < 0.001), showing high specificity (85.16 %), sensitivity (78.62 %), and negative predictive value (NPV) (95.08 %) at the optimal cut-off. This algorithm resulted in fewer patients with indeterminate results compared to ELF. Its diagnostic performance in the two external validation cohorts was comparable to that in the derivation cohort.Conclusions
The TaP score demonstrated good diagnostic ability with generally better performance compared to ELF, and had the potential to be developed as a novel NIT.
基于蛋白质生物标志物的晚期肝纤维化风险预测和诊断新算法的开发和临床验证:一项多中心研究
2型糖尿病(T2D)和肥胖显著增加代谢功能障碍相关脂肪变性肝病(MASLD)患者肝纤维化的风险。然而,目前还缺乏一种可靠且具有成本效益的非侵入性检测方法(NIT)来检测T2D/肥胖患者的肝纤维化。本研究旨在开发一种简单的基于生物标志物的算法,用于检测T2D/肥胖合并MASLD患者的晚期肝纤维化,并验证其在临床和社区队列中的诊断性能。方法在三个独立的队列中评估循环血小板反应蛋白-2 (TSP2)(一种新型纤维化标志物)和增强肝纤维化(ELF)试验的三个单独成分的诊断性能。其中包括基于临床的衍生队列(N = 846)和基于社区的验证队列(N = 803),均由振动控制瞬态弹性成像(VCTE)诊断为MASLD的T2D患者组成。此外,纳入了一个基于临床的验证队列,该队列包括活检证实的MASLD的病态肥胖患者(N = 223)。基于多变量logistic回归模型构建基于TSP2和前胶原3 n端肽(PIIINP) (ELF的一个组成部分)的算法(TaP评分),并与现有的nit(包括ELF、纤维化-4指数(FIB-4)和NAFLD纤维化评分(NFS)进行比较。采用双截止方法确定规则输入和排除截止。结果循环TSP2 (AUC[95 %CI]:0.844[0.810-0.878])和PIIINP (AUC[95 %CI]:0.843(0.807-0.875])具有较好的诊断效果,可用于构建基于生物标志物的诊断算法。水龙头评分(AUC(95 % CI): 0.900[0.874 - -0.925])明显优于精灵(AUC[95 % CI]: 0.809 [0.773 - -0.843]), FIB-4 (AUC[95 % CI]: 0.597[0.544 - -0.647])和NFS (AUC(95 % CI): 0.585(0.528 - -0.639))(所有的德龙P & lt; 0.001),显示高特异性(85.16 %),灵敏度(78.62 %),和负面预测值(NPV)(95.08 %)最优截止。与ELF相比,该算法导致结果不确定的患者较少。它在两个外部验证队列中的诊断性能与衍生队列中的诊断性能相当。结论TaP评分具有较好的诊断能力,总体上优于ELF评分,具有发展为新型NIT的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


