HEBP2-governed glutamine competition between tumor and macrophages dictates immunotherapy efficacy in triple-negative breast cancer
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Immunotherapy demonstrates limited efficacy in triple-negative breast cancer (TNBC), influenced by intricate metabolic interactions within the tumor microenvironment. Here, we developed a single-cell RNA sequencing (scRNA-seq) immunotherapy cohort (N = 27) and a spatial transcriptomics cohort (N = 88) to elucidate metabolic crosstalk associated with therapeutic efficacy in TNBC. We illustrated that heme binding protein 2 (HEBP2)high tumor cells (featured by active glutathione metabolism) and CCL3+ macrophages (characterized by oxidative metabolism) indicated immunotherapy efficacy and were quantitatively and spatially negatively correlated. HEBP2-mediated glutamine face-off between these cell types induced this phenomenon. Mechanistically, HEBP2 disrupted FOXA1 cytoplasmic phase separation, promoting its nuclear translocation to upregulate glutathione S-transferase P1 (GSTP1) expression and glutamine consumption in tumor cells. This metabolic shift induced ferroptosis of CCL3+ macrophages, impairing the antitumor immunity. The utilization of a GSTP1 inhibitor sensitized TNBC to immunotherapy. Collectively, we delineate a tumor-macrophage metabolic checkpoint governed by the HEBP2/GSTP1 axis and pioneer single-cell-level immunometabolism as a paradigm for evaluating immunotherapeutic vulnerabilities.
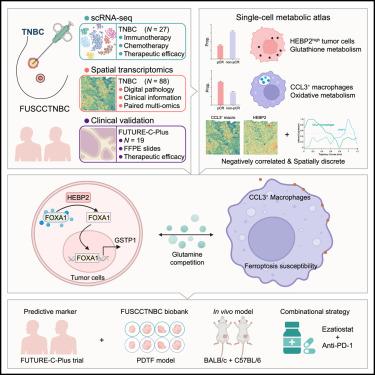
hebp2调控的肿瘤和巨噬细胞之间的谷氨酰胺竞争决定了三阴性乳腺癌的免疫治疗效果
免疫疗法对三阴性乳腺癌(TNBC)的疗效有限,受肿瘤微环境中复杂的代谢相互作用的影响。在这里,我们建立了单细胞RNA测序(scRNA-seq)免疫治疗队列(N = 27)和空间转录组学队列(N = 88)来阐明与TNBC治疗效果相关的代谢串扰。我们发现血红素结合蛋白2 (HEBP2)高的肿瘤细胞(以谷胱甘肽代谢活跃为特征)和CCL3+巨噬细胞(以氧化代谢为特征)表明免疫治疗效果,并在数量和空间上呈负相关。hebp2介导的谷氨酰胺在这些细胞类型之间的对峙诱导了这一现象。从机制上讲,HEBP2破坏FOXA1细胞质相分离,促进其核易位,上调谷胱甘肽s -转移酶P1 (GSTP1)的表达和谷氨酰胺的消耗。这种代谢转变诱导CCL3+巨噬细胞铁下垂,损害抗肿瘤免疫。使用GSTP1抑制剂使TNBC对免疫治疗增敏。总之,我们描述了一个由HEBP2/GSTP1轴控制的肿瘤-巨噬细胞代谢检查点,并开创了单细胞水平免疫代谢作为评估免疫治疗脆弱性的范例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


