Systematic Assessment of the Structural Impact of Linker Fluorination in CeIV-Based UiO-66
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
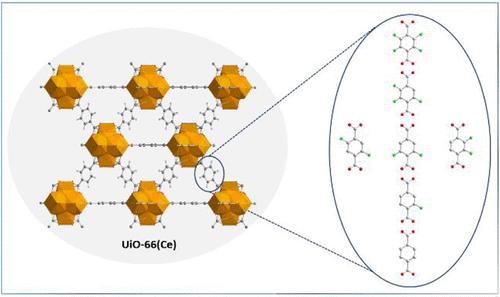
Incorporating fluorine atoms into the organic linker is a widely adopted strategy to tune the properties of Metal–Organic Frameworks (MOFs). However, identifying a trend in how fluorine atoms modulate the structure of porous frameworks remains an unaddressed topic. We report a systematic investigation of how stepwise fluorination of 1,4-benzenedicarboxylic acid linkers (Fx-H2BDC) influences the structural features of the CeIV-based UiO-66 architecture. The complete series of fluorinated Fx_UiO-66(Ce) materials (where x = 0, 1, 2, 3, 4) was synthesized using acetic acid as a crystallization modulator in a mixed DMF:H2O solvent and fully characterized. 1H and 19F liquid-state NMR spectroscopy of digested samples, supported by thermogravimetric analysis, confirmed that all fluorinated derivatives are defect-free, whereas the nonfluorinated analogue contains a small amount of missing-linker defects. N2 adsorption measurements revealed a decreasing trend in BET surface areas with increasing fluorination. Rietveld refinements of high-resolution synchrotron powder X-ray diffraction data indicate a gradual expansion of the unit cell as the degree of fluorination increased, along with the elongation of the CeIV–Ocarboxylate bonds, while the aromatic rings are progressively twisted out of the plane of the carboxylates and a water molecule is incorporated into the coordination sphere of CeIV.
ceiv基UiO-66中连接剂氟化对结构影响的系统评价
在有机连接体中加入氟原子是一种广泛采用的调整金属-有机框架(mof)性能的策略。然而,确定氟原子如何调节多孔框架结构的趋势仍然是一个未解决的主题。我们系统地研究了1,4-苯二甲酸连接剂(Fx-H2BDC)的逐步氟化如何影响基于ceiv的UiO-66结构的结构特征。在DMF:H2O混合溶剂中,以乙酸为结晶调节剂合成了全系列氟化fx_ueo -66(Ce)材料(其中x = 0,1,2,3,4),并对其进行了表征。消化样品的1H和19F液态核磁共振波谱,在热重分析的支持下,证实所有氟化衍生物都是无缺陷的,而非氟化类似物含有少量缺失连接体缺陷。N2吸附测量显示BET表面积随氟化程度的增加呈下降趋势。高分辨率同步加速器粉末x射线衍射数据的Rietveld改进表明,随着氟化程度的增加,随着CeIV -羧酸盐键的延长,单位细胞逐渐扩大,同时芳香环逐渐扭曲出羧酸盐平面,一个水分子被纳入CeIV的配位球。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


