A Defective PtCoO2 2D Conductor with Resistivity Lower than Pt: Design of Molten Salt Synthesis
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Highly defective 2D layered molten salt synthesized polycrystalline PtCoO2 is metallic down to 1.8 K with a resistivity of 1.36 ± 0.05 μΩ·cm at 300 K, which is an order lower than that of Pt. The design of NaCl–CoCl2 molten salt and its chemistry for obtaining phase-pure PtCoO2 are presented. Dynamic changes in liquidus temperatures brought about by the compositional changes of NaCl–CoCl2 upon consumption of CoCl2 during the reaction are constrained to be within ±35 °C by a predesigned salt mixture. Rietveld structural refinements of XRD and SAED confirm the R3̅m space group with predominant (00l) texturing. XPS reveals that Co and Pt are predominantly in +3 and +1 oxidation states, respectively. PtCoO2 is paramagnetic due to the low-spin Co4+, which undergoes antiferromagnetic ordering around 14 K. The catalytic property of PtCoO2 is investigated for H2 sensing.
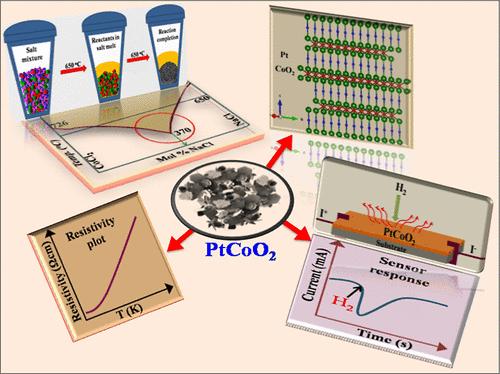
电阻率低于Pt的缺陷PtCoO2二维导体:熔盐合成的设计
高缺陷二维层状熔盐合成的多晶PtCoO2在300 K时的电阻率为1.36±0.05 μΩ·cm,低于Pt的一个级。本文介绍了NaCl-CoCl2熔盐的设计及其制备相纯PtCoO2的化学过程。通过预先设计的盐混合物,将反应过程中消耗CoCl2时NaCl-CoCl2组分变化引起的液相温度动态变化限制在±35℃以内。XRD和SAED的Rietveld结构细化证实了以(00l)织构为主的R3 _ m空间群。XPS显示Co和Pt分别以+3和+1氧化态为主。PtCoO2是顺磁性的,这是由于低自旋的Co4+,它在14k左右经历反铁磁有序。研究了PtCoO2对H2传感的催化性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


