A Charge-Transfer Salt Featuring a High-Entropy and Plastic Crystal
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
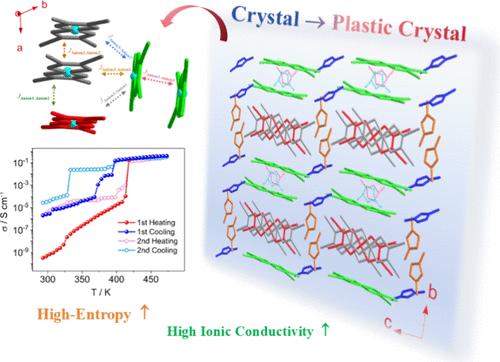
Ionic plastic crystals (IPCs) bridge the structural rigidity of crystalline solids and the dynamic flexibility of liquids featuring partially disordered molecular orientations within an ordered translational lattice. Herein, we report the charge-transfer salt [DMIm][Ni(mnt)2] (1; [DMIm]+ = 1,3-dimethylimidazolium, mnt2– = maleonitriledithiolate), which undergoes a crystal-to-plastic crystal phase transition at 414 K upon heating. In the low-temperature crystalline phase, 1 crystallizes in the triclinic space group P-1. Its asymmetric unit contains three distinct ion-pairs, comprising six crystallographically independent components. The [Ni(mnt)2]− anion radicals form one-dimensional stacks, interconnected via [Ni(mnt)2]− dimers into two-dimensional layers. This layered structure leads to anisotropic dielectric permittivity along directions parallel and perpendicular to the layers. Three crystallographically distinct cations occupy voids within and between these layers. The presence of multiple crystallographically independent components confers a high configurational entropy on 1, reflected in a large entropy change during its phase transition. 1 also exhibits superior ionic conductivity, reaching 0.42 S cm–1 in its plastic crystal phase. Magnetic analysis reveals strong antiferromagnetic interactions between neighboring anions, rendering 1 nearly diamagnetic at room temperature. This study provides insights for designing multifunctional molecular materials that integrate high entropy, ionic conductivity, and tailored magnetic properties.
一种具有高熵和塑性晶体的电荷转移盐
离子塑料晶体(IPCs)连接了晶体固体的结构刚性和在有序的平移晶格中具有部分无序分子取向的液体的动态柔韧性。在此,我们报道了电荷转移盐[DMIm][Ni(mnt)2] (1; [DMIm]+ = 1,3-二甲基咪唑,mmn2 - =马来硝基二硫酸盐),该盐在414 K加热后经历了晶体到塑性晶体的相变。在低温晶相中,1在三斜空间群P-1中结晶。它的不对称单元包含三个不同的离子对,由六个晶体学上独立的组分组成。[Ni(mnt)2]−阴离子自由基形成一维堆叠,通过[Ni(mnt)2]−二聚体相互连接成二维层。这种层状结构导致沿平行和垂直于层的方向的各向异性介电常数。三种晶体学上不同的阳离子占据了这些层内部和层之间的空隙。多个晶体独立组分的存在赋予了1上的高构型熵,反映在其相变过程中的大熵变。1也表现出优异的离子电导率,在其塑性晶相中达到0.42 S cm-1。磁性分析揭示了相邻阴离子之间的强反铁磁相互作用,使得1在室温下几乎是抗磁性的。该研究为设计集成高熵、离子电导率和定制磁性质的多功能分子材料提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


