Stepwise 6H+/6e– Electron-Coupled Proton Buffers Based on Fe and Redox-Active Ligands
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
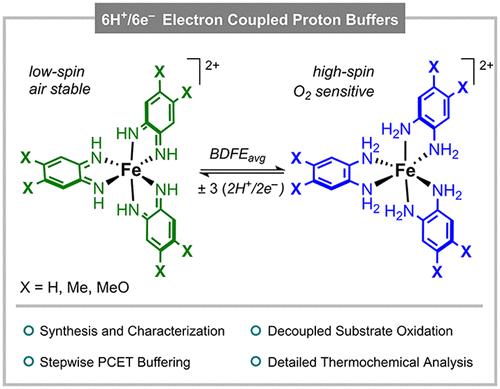
Herein, we report electron-coupled-proton buffers (ECPBs) based on Fe and redox-active ortho-phenylenediamine (opda) ligands that perform stepwise and reversible 6H+/6e– transformations. Four of the Fe complexes involved in the PCET transformation (namely X62+, X8H22+, X10H42+ and X12H62+) were structurally and/or spectroscopically characterized. The reductive protonation of X62+ to X12H62+ and the oxidative deprotonation of X12H62+ to X62+ were carried out using PCET reagents, which indicate that these 6H+/6e– transformations occurred in a 2H+/2e– fashion, accumulating the intermediate species X8H22+and X10H42+. The thermochemistry of the 2H+/2e– and overall 6H+/6e– transformations was studied by open-circuit potential measurements and comproportionation reactions. Interestingly, the Fe-based ECPBs depicted redox unleveling, in which the average bond dissociation free energy (BDFEavg) of the 2H+/2e– reductive protonation of X62+ to X8H22+ was substantially higher than the BDFEavg of the 6H+/6e– conversion of X62+ to X12H62+. We also show that the BDFEavg of the PCET transformations involving the Fe system bearing unsubstituted opda are higher than the systems bound by 4,5-Me2-opda and 4,5-(MeO)2-opda, a manifestation of redox decompensation. The capability of the Fe-based ECPBs to accept and donate H-atom equivalents, as well as their ability to dehydrogenate organic substrates using O2 as oxidant in a decoupled fashion, was also evaluated.
基于Fe和氧化还原活性配体的6H+/6e -电子耦合质子缓冲液
在此,我们报道了基于铁和氧化还原活性邻苯二胺(opda)配体的电子偶质子缓冲液(ECPBs),它们可以进行逐步可逆的6H+/6e -转化。参与PCET转化的四种Fe配合物(即X62+, X8H22+, X10H42+和X12H62+)进行了结构和/或光谱表征。用PCET试剂进行了X62+还原质子化成X12H62+和X12H62+氧化去质子化成X62+的实验,结果表明,这些6H+/6e -转化是以2H+/2e -的方式发生的,中间产物X8H22+和X10H42+积累而成。通过开路电位测量和比例反应研究了2H+/2e -和总体6H+/6e -转化的热化学性质。有趣的是,基于铁的ECPBs描述了氧化还原不平衡,其中X62+的2H+/2e -还原质子化到X8H22+的平均键解离自由能(BDFEavg)大大高于X62+到X12H62+的6H+/6e -转化的BDFEavg。我们还发现含有未取代opda的Fe体系的PCET转换的BDFEavg高于由4,5- me2 -opda和4,5-(MeO)2-opda结合的体系,这是氧化还原失代偿的表现。研究还评估了铁基ECPBs接受和提供h原子等价物的能力,以及它们以去耦方式使用O2作为氧化剂使有机底物脱氢的能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


