Therapeutic Plasma Exchange in Patients With Acute Liver Failure: A Pilot Randomized Controlled Trial
IF 3.2
Q2 GASTROENTEROLOGY & HEPATOLOGY
Journal of Clinical and Experimental Hepatology
Pub Date : 2025-08-26
DOI:10.1016/j.jceh.2025.103178
引用次数: 0
Abstract
Background/Aims
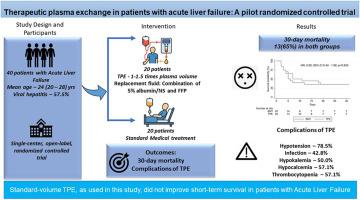
Acute liver failure (ALF) is a life-threatening condition with high short-term mortality without liver transplantation (LT). Prior studies on therapeutic plasma exchange (TPE) have yielded mixed survival results. In this randomized controlled trial (RCT), we evaluated the safety and efficacy of TPE on the 30-day outcomes in ALF.
Methods
In this single-center, open-label, pilot RCT, 40 adults with ALF were randomized in a 1:1 ratio to receive either standard medical therapy (SMT) alone or SMT plus TPE. TPE was performed daily for up to five days or stopped as per the predefined criteria (improvement in hepatic encephalopathy or the development of shock or sepsis), using standard-volume centrifugation-based with a 1:1 fresh frozen plasma and albumin replacement. The primary outcome was 30-day mortality. Secondary outcomes included changes in biochemical parameters and adverse events.
Results
Median age was 24 (20-28) years; 57.5% were female; 57.5% had viral hepatitis–related ALF. Among TPE-assigned patients, 14 of 20 received ≥1 sessions. Thirty-day mortality was identical in both arms (65%; intention-to-treat, P = 1.000), with similar results on per-protocol analysis (TPE: 78.6% vs SMT: 65.0%, P = 0.393). By day 3, TPE recipients showed greater reductions in bilirubin (P = 0.002), international normalized ratio (P = 0.013), and ammonia (P = 0.340), but survival was unchanged. Rates of infection and electrolyte disturbances were similar. TPE was not independently associated with survival (hazard ratio: 0.92, 95% confidence interval [CI]: 0.43-1.99, P = 0.83).
Conclusion
In our study, standard-volume TPE led to transient biochemical improvements but did not improve 30-day transplant-free survival. Future large, adequately powered multicentric RCTs are needed to clarify the potential role of TPE in ALF. This trial was prospectively registered with the Clinical Trials Registry of India (CTRI/2017/08/009525).
血浆置换治疗急性肝衰竭患者:一项随机对照试验
背景/目的急性肝衰竭(ALF)是一种危及生命的疾病,短期死亡率高,无需肝移植(LT)。先前的治疗性血浆交换(TPE)研究得出了不同的生存结果。在这项随机对照试验(RCT)中,我们评估了TPE对ALF患者30天预后的安全性和有效性。方法在这项单中心、开放标签、先导随机对照试验中,40名ALF成人患者按1:1的比例随机分为单独接受标准药物治疗(SMT)或SMT加TPE两组。TPE每天进行5天,或根据预先确定的标准(肝性脑病的改善或休克或败血症的发展)停止,使用标准体积离心为基础的1:1新鲜冷冻血浆和白蛋白替代。主要终点为30天死亡率。次要结局包括生化参数的改变和不良事件。结果患者中位年龄24岁(20 ~ 28岁);57.5%为女性;57.5%为病毒性肝炎相关ALF。在tpe分配的患者中,20名患者中有14名接受了≥1次治疗。两组的30天死亡率相同(65%;意向治疗,P = 1.000),每个方案分析的结果相似(TPE: 78.6% vs SMT: 65.0%, P = 0.393)。到第3天,TPE患者胆红素(P = 0.002)、国际标准化比值(P = 0.013)和氨(P = 0.340)均有较大下降,但生存率不变。感染和电解质紊乱的发生率相似。TPE与生存率无独立相关性(风险比:0.92,95%可信区间[CI]: 0.43-1.99, P = 0.83)。结论在我们的研究中,标准体积TPE导致短暂的生化改善,但没有提高30天无移植生存。未来需要更大规模、更有力的多中心随机对照试验来阐明TPE在ALF中的潜在作用。该试验已在印度临床试验注册中心(CTRI/2017/08/009525)前瞻性注册。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Clinical and Experimental Hepatology
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
4.90
自引率
16.70%
发文量
537
审稿时长
64 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


