Efficient and improved synthesis of kukoamine D and N1,N5,N14-tris(dihydrocaffeoyl)spermine
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Natural products kukoamine D and N1,N5,N14-tris(dihydrocaffeoyl)spermine, two hydrocaffeoylspermine alkaloids predominantly isolated from Solanaceae plants, exhibit diverse pharmacological activities. However, low natural contents and inefficient synthesis methods limit their applications. Commencing with tert-butyloxycarbonyl (Boc)-protected 1,4-butanediamine, we synthesized kukoamine D and N1,N5,N14-tris(dihydrocaffeoyl)spermine via an eight-step sequence, achieving overall yields of 29 % and 20 %, respectively. We used a one-pot two-step, nitrile reductive amination method using a nickel chloride (NiCl2·6H2O)/sodium borohydride (NaBH4) system, followed by active esters amidation. The synthesis is simple, cost effective, and scalable. This approach provides a practical route for the industrial production of structurally similar natural products, facilitating pharmacological studies and analog development.
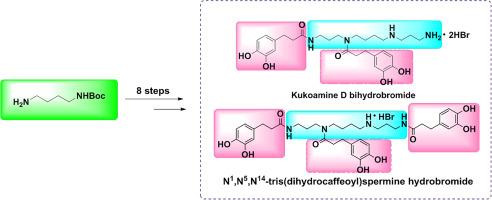
kukoamine D和N1,N5, n14 -三(二氢咖啡因基)精胺的高效改进合成
天然产物kukoamine D和N1,N5,N14-tris(二氢咖啡因基)精胺是两种主要从茄科植物中分离出来的氢咖啡因基精胺生物碱,具有多种药理活性。然而,天然含量低和合成方法效率低限制了其应用。从叔丁基羰基(Boc)保护的1,4-丁二胺开始,经过8步合成了kukoamine D和N1,N5,N14-tris(二氢咖啡因基)精胺,总产率分别为29%和20%。采用氯化镍(NiCl2·6H2O)/硼氢化钠(NaBH4)体系进行一锅两步腈还原胺化,然后进行活性酯化。这种合成方法简单、经济、可扩展。这种方法为结构相似的天然产物的工业生产提供了一条实用的途径,促进了药理学研究和类似物的开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


