Fabrication and application of iron oxide-encapsulated PLGA nanoparticles with dual responsiveness to magnetic fields and light for nose-to-brain drug delivery
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-15
DOI:10.1016/j.jddst.2025.107535
引用次数: 0
Abstract
Nose-to-brain delivery has been widely investigated as a potential strategy for glioma therapy. However, the nasal epithelial barrier remains a major obstacle to drug transport from the nasal cavity to the brain, particularly for macromolecular agents such as peptides, nucleic acids, and nanoparticles. Therefore, strategies to enhance epithelial permeability are required. In this study, we developed a drug delivery system to improve nose-to-brain transport through transcranial magnetic field application, with the aim of contributing to glioma treatment. Iron oxide nanoparticles (IONPs), which possess both superparamagnetic and photothermal properties, were utilized to enhance brain penetration and to enable photothermal therapy (PTT). IONPs were encapsulated in poly(lactic-co-glycolic acid) (PLGA) nanoparticles to form IONPs@PLGA, with an average size of approximately 200 nm. Transmission electron microscopy revealed that IONPs were located inside PLGA nanoparticles, and laser irradiation (660 nm) raised the temperature to 50 °C, suggesting that IONPs@PLGA generated sufficient heat to induce cancer cell death. Moreover, IONPs@PLGA were efficiently internalized by cells under a magnetic field, and laser irradiation induced strong cytotoxicity against C6 glioma cells. Notably, applying a magnetic field after intranasal administration increased brain accumulation by ∼2.5-fold, confirming enhanced delivery via magnetic targeting. In summary, we developed IONPs@PLGA, a dual magnetic- and light-responsive system, and demonstrated its potential to improve nose-to-brain delivery. Given their drug-loading capacity, IONPs@PLGA represent a promising platform for magnetically guided, non-invasive brain drug delivery.
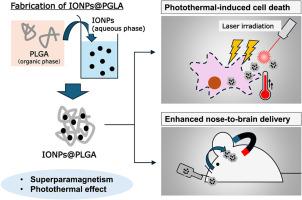
具有磁场和光双重响应的氧化铁封装PLGA纳米颗粒的制备和应用
鼻到脑输送作为神经胶质瘤治疗的一种潜在策略已被广泛研究。然而,鼻腔上皮屏障仍然是药物从鼻腔转运到大脑的主要障碍,特别是对于大分子药物,如肽、核酸和纳米颗粒。因此,需要提高上皮通透性的策略。在这项研究中,我们开发了一种药物输送系统,通过经颅磁场应用来改善鼻到脑的运输,目的是为胶质瘤的治疗做出贡献。氧化铁纳米颗粒(IONPs)具有超顺磁性和光热性质,可用于增强脑穿透和光热治疗(PTT)。IONPs包被聚乳酸-羟基乙酸(PLGA)纳米粒子形成IONPs@PLGA,平均尺寸约为200 nm。通过透射电镜观察发现,IONPs位于PLGA纳米颗粒内部,激光照射(660 nm)将温度提高到50℃,表明IONPs@PLGA产生了足够的热量诱导癌细胞死亡。此外,IONPs@PLGA在磁场作用下被细胞有效内化,激光照射对C6胶质瘤细胞具有较强的细胞毒性。值得注意的是,在鼻内给药后施加磁场使脑积聚增加了约2.5倍,证实了通过磁靶向增强给药。总之,我们开发了IONPs@PLGA,一个双磁和光响应系统,并证明了其改善鼻子到大脑传递的潜力。考虑到它们的药物装载能力,IONPs@PLGA代表了一个有前途的平台,用于磁引导,非侵入性脑药物输送。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


