Electrospun mucoadhesive nanofibrous films for intranasal delivery of propranolol hydrochloride for migraine prophylaxis
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-19
DOI:10.1016/j.jddst.2025.107552
引用次数: 0
Abstract
Migraine treatment failure is often associated with the delayed onset and variable efficacy of conventional oral medications, which are further limited by poor patient compliance due to gastrointestinal side effects and frequent dosing requirements. To address these challenges, a fast-disintegrating mucoadhesive nanofibrous film was developed for the intranasal administration of propranolol hydrochloride (PROP), a well-established migraine prophylactic agent. The electrospun films were formulated using rapidly dissolving pullulan and chitosan, known for its strong mucoadhesive properties, ensuring immediate drug availability at the site of action and promoting permeation through the nasal epithelium via the nose-to-brain delivery route, thereby bypassing first-pass metabolism. The films demonstrated sufficient elasticity, allowing them to be cut and rolled into solid dosage forms for convenient intranasal administration. Upon hydration, the films transitioned into a gel-like state, as confirmed by rheological studies conducted in simulated nasal electrolyte solution (SNES) at 35 °C. In vitro release studies in SNES revealed rapid PROP dissolution, while cytocompatibility was assessed using the Calu-3 cell line, showing dose-dependent effects likely influenced by the PROP concentration. Mucoadhesion studies indicated a significant enhancement of retention at the nasal mucosa, with higher chitosan concentrations contributing to improved drug residence and facilitated transport across the epithelium. To overcome the limitations of oral PROP administration, such as poor bioavailability and delayed onset of action, the proposed electrospun nanofiber films might offer a promising alternative for effective and patient-friendly migraine therapy via intranasal delivery.
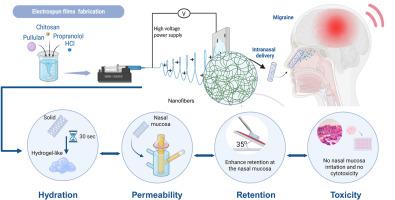
电纺丝黏附纳米纤维膜用于预防偏头痛的鼻内给药盐酸心得安
偏头痛治疗失败通常与传统口服药物的延迟发作和疗效不一有关,由于胃肠道副作用和频繁的给药要求,患者依从性较差,这进一步受到限制。为了解决这些挑战,研究人员开发了一种快速崩解的黏附纳米纤维薄膜,用于鼻内给药盐酸心得安(propranolol hydrochloride, PROP),一种公认的偏头痛预防药物。这种电纺丝薄膜是用快速溶解的普鲁兰和壳聚糖配制的,壳聚糖以其强大的黏附特性而闻名,确保药物在作用部位立即可用,并通过鼻子到大脑的给药途径促进通过鼻上皮的渗透,从而绕过第一过代谢。薄膜表现出足够的弹性,允许它们被切割和卷成固体剂型,方便鼻内给药。在35°C的模拟鼻电解质溶液(SNES)中进行的流变学研究证实,水化后,膜转变为凝胶状状态。SNES的体外释放研究显示PROP快速溶解,而使用Calu-3细胞系评估细胞相容性,显示可能受PROP浓度影响的剂量依赖性效应。黏附研究表明,高浓度的壳聚糖有助于改善药物在鼻黏膜的滞留,促进药物在上皮间的运输。为了克服口服PROP给药的局限性,如生物利用度差和起效延迟,所提出的电纺丝纳米纤维薄膜可能通过鼻内给药为有效和患者友好的偏头痛治疗提供一个有希望的替代方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


