Design, synthesis and herbicidal activity of novel 2,4-dichlorobenzene-3-morpholine derivatives as potential hydroxyphenylpyruvate dioxygenase inhibitors
IF 4
1区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
4-Hydroxyphenylpyruvate dioxygenase (HPPD, EC 1.13.11.27) is a key target for herbicide development, especially in the management of herbicide-resistant weeds. However, many existing HPPD inhibitors suffer from poor crop selectivity, limiting their widespread application. To address this problem, a series of novel 2,4-dichlorobenzene-3-morpholine derivatives were designed and synthesized. Greenhouse activity tests demonstrated that a large class of molecules obviously inhibited dwarfing in the tested weeds. Notably, compound B2 exhibited superior herbicidal activity against broadleaf weeds, such as Abutilon theophrasti, Zinnia elegans, and Portulaca oleracea, at 9.375 g ai/ha, which is superior to that of the widely used herbicide mesotrione. Moreover, compared with mesotrione, compound B2 showed no obvious injury on the growth of peanuts at 150 g ai/ha and was safer for peanuts, even at the higher dosage of 600 g ai/ha. These results suggest that B2 is a promising new candidate herbicide for use in peanut fields. Molecular simulations of the representative molecule further revealed a binding mode that fit into the active site of HPPD, similar to most known HPPD inhibitors. Met338 in P. oleracea HPPD plays a crucial role in the binding of compound B2, whereas Ile453 and Ile455 in peanut HPPD may contribute to reduced inhibition in peanuts. Additionally, the entry pathways of compounds, along with their polarity and lipophilicity, could increase their selectivity for different plant species. This study provides valuable insights for the future development of highly selective HPPD inhibitors.
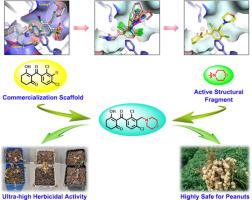
新型羟基苯基丙酮酸双加氧酶抑制剂2,4-二氯苯-3-啉衍生物的设计、合成及除草活性研究
4-羟基苯基丙酮酸双加氧酶(HPPD, EC 1.13.11.27)是除草剂开发的关键靶点,特别是在抗除草剂杂草管理中。然而,许多现有的HPPD抑制剂存在作物选择性差的问题,限制了它们的广泛应用。为了解决这一问题,设计并合成了一系列新的2,4-二氯苯-3-啉衍生物。温室活性试验表明,一大类分子明显抑制了被试杂草的矮化。值得注意的是,化合物B2对阔叶杂草(Abutilon theophrasti)、百日草(Zinnia elegans)和马齿苋(Portulaca oleracea)的除草活性为9.375 g /ha,优于目前广泛使用的除草剂美索三酮。此外,与美索三酮相比,在150 g ai/ha时,化合物B2对花生的生长没有明显的伤害,即使在600 g ai/ha的较高剂量下,化合物B2对花生也更安全。这些结果表明,B2是一种很有前景的花生田除草剂。代表性分子的分子模拟进一步揭示了一种适合HPPD活性位点的结合模式,类似于大多数已知的HPPD抑制剂。在甘蓝HPPD中,me338在化合物B2的结合中起关键作用,而在花生HPPD中,Ile453和Ile455可能有助于降低花生对化合物B2的抑制。此外,化合物的进入途径以及它们的极性和亲脂性可以增加它们对不同植物物种的选择性。该研究为未来开发高选择性HPPD抑制剂提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.00
自引率
8.50%
发文量
238
审稿时长
4.2 months
期刊介绍:
Pesticide Biochemistry and Physiology publishes original scientific articles pertaining to the mode of action of plant protection agents such as insecticides, fungicides, herbicides, and similar compounds, including nonlethal pest control agents, biosynthesis of pheromones, hormones, and plant resistance agents. Manuscripts may include a biochemical, physiological, or molecular study for an understanding of comparative toxicology or selective toxicity of both target and nontarget organisms. Particular interest will be given to studies on the molecular biology of pest control, toxicology, and pesticide resistance.
Research Areas Emphasized Include the Biochemistry and Physiology of:
• Comparative toxicity
• Mode of action
• Pathophysiology
• Plant growth regulators
• Resistance
• Other effects of pesticides on both parasites and hosts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


