Engineering Cu(OH)₂-AgNP@MWCNT nanocomposite by systematically layering onto a glassy carbon substrate and application as an electrochemical sensor for the analysis of antidiabetic agent metformin
IF 5.1
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
Abstract
In this study, a novel electrode was engineered by systematically layering a Cu(OH)₂-AgNP@MWCNT nanocomposite onto a glassy carbon substrate. The Cu(OH)2-AgNP@MWCNT electrode is a sensitive electrochemical sensor designed for the selective detection of metformin, a drug approved by the FDA, primarily used for managing high blood sugar levels in individuals with type 2 diabetes. It is applicable in both clinical and pharmaceutical environments. The characterization of the components was performed utilizing Fourier-transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), and X-ray diffraction (XRD). All electrochemical assessments were conducted utilizing cyclic voltammetry (CV), electrochemical impedance spectrometry (EIS), and differential pulse voltammetry (DPV). The electroactive surface area (ECSA) of the working electrode was determined, measuring 0.574 cm2 compared to just 0.127 cm2 for the unmodified electrode, suggesting greater efficiency and enhanced performance. The sensor exhibited two linear responses for metformin concentrations ranging from 0.02 to 2.0 μM and 2.0 to 100 μM, with limits of detections (LODs) established at 0.014 and 1.52 μM, respectively, and was successfully applied to the determination of metformin in real samples, yielding satisfactory results. The newly developed electrode's performance was evaluated in comparison to the HPLC method using a statistical F-test (P = 0.01). The findings demonstrated that the recovery rates from both methods were comparable and highlighted that the electrode's precision in response actually provides better accuracy than the HPLC technique for analyzing metformin in samples.
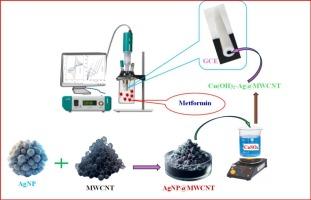
工程Cu(OH)₂-AgNP@MWCNT纳米复合材料,系统地分层在玻碳衬底上,并作为电化学传感器用于分析降糖药二甲双胍
在这项研究中,通过系统地将Cu(OH)₂-AgNP@MWCNT纳米复合材料分层到玻璃碳衬底上,设计了一种新型电极。Cu(OH)2-AgNP@MWCNT电极是一种灵敏的电化学传感器,专为选择性检测二甲双胍而设计,二甲双胍是一种经FDA批准的药物,主要用于控制2型糖尿病患者的高血糖水平。它适用于临床和制药环境。利用傅里叶变换红外光谱(FT-IR)、扫描电子显微镜(SEM)、能量色散x射线光谱(EDX)和x射线衍射(XRD)对组分进行表征。所有电化学评估均采用循环伏安法(CV)、电化学阻抗谱法(EIS)和差分脉冲伏安法(DPV)进行。测定了工作电极的电活性表面积(ECSA),测量值为0.574 cm2,而未修饰电极的ECSA仅为0.127 cm2,表明效率更高,性能增强。该传感器对二甲双胍浓度在0.02 ~ 2.0 μM和2.0 ~ 100 μM范围内具有线性响应,检出限分别为0.014 μM和1.52 μM,可用于实际样品中二甲双胍的检测,结果令人满意。采用统计f检验(P = 0.01)对新电极的性能与HPLC法进行比较。结果表明,两种方法的回收率具有可比性,并强调电极的响应精度实际上比HPLC技术在分析样品中的二甲双胍方面提供了更好的准确性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


