Influence of stress on physical properties of CsGaO3 for enhanced photocatalytic application: A DFT perspective
IF 4.9
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
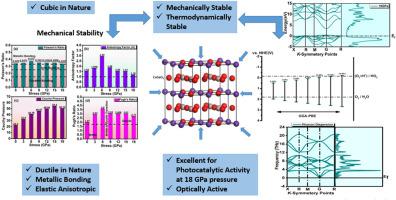
The dual threat of water contamination and energy resource depletion has prompted increased research into hydrogen as a green energy source. However, its evolution and storage remain problematic. To address this, researchers are focusing on perovskite hydrides, known for their strong ion exchange abilities and impressive storage capacity by weight. Dealing with severe energy crisis and water contamination, we have performed the calculations for our considered compound CsGaO3 at different pressure from 0 GPa to 18 GPa by employing the GGA-PBE approach by using first principles computational method, using density functional theory (DFT) via CASTEP to address this issue. We have computed the structural, photocatalytic, electronic, optical, mechanical, phononic and thermodynamic properties of CsGaO3 under the considered stress range of 0 GPa–18GPa. From the structural analysis it is confirmed that no phase transition occurs and CsGaO3 possess cubic behavior at all the pressures. The thermodynamic sustainability of our considered material is confirmed by the negative formation energy, free energy, entropy and heat capacity etc. Under considered stress a continuous decrease in lattice constants and an increase in band gap (1.501eV–2.904eV) is inspected up to 18 GPa. For a thorough assessment of band gap, EPDOS and TDOS have also been conclude. It is concluded from the electronic properties that at 18Gpa, our tuned band gap is most suitable for the photocatalytic water splitting application in visible range under solar radiations. For quick response to incoming photons, good absorption and extinction coefficient are key parameters. From calculated optical parameters we come to know that our compound at considered pressure possess excellent absorptive properties for its utilization in photocatalysis. Its mechanical stability is confirmed by the elastic constants in GPa. We come to know from the estimated parameters that, brittleness decline as the pressure is being applied and our compound possess ductile nature at considered pressure which increases its impact for the application of photocatalytic water splitting. Anisotropic nature of CsGaO3 is observed in anticipated outcomes. At the end we have calculated phonon dispersion curve and density of state to confirm its dynamical stability. According to our computed results, our compound is perfectly dynamically stable which provides an exceptional favor to use it in photocatalytic water splitting application and hydrogen evolution.
应力对cscao3增强光催化性能的影响:DFT视角
水污染和能源枯竭的双重威胁促使氢作为一种绿色能源的研究日益增加。然而,它的进化和储存仍然存在问题。为了解决这个问题,研究人员将重点放在钙钛矿氢化物上,钙钛矿氢化物以其强大的离子交换能力和令人印象深刻的重量存储容量而闻名。为了解决严重的能源危机和水污染问题,我们采用GGA-PBE方法,采用第一性原理计算方法,利用CASTEP中的密度泛函理论(DFT),在0 ~ 18 GPa的不同压力下对我们所考虑的化合物cs高3进行了计算。在0 GPa-18GPa的应力范围内,计算了cscao3的结构、光催化、电子、光学、力学、声子和热力学性质。结构分析证实,cscao3在所有压力下均不发生相变,具有立方态行为。我们所考虑的材料的热力学可持续性由负的形成能、自由能、熵和热容等来证实。在考虑的应力下,晶格常数持续下降,带隙增加(1.501eV-2.904eV),直至18 GPa。为了全面地评估带隙,还总结了EPDOS和TDOS。从电子性质上得出,在18Gpa时,我们的调谐带隙最适合在太阳辐射可见光范围内的光催化水分解应用。为了快速响应入射光子,良好的吸收和消光系数是关键参数。从计算得到的光学参数可知,该化合物在一定压力下具有良好的光催化吸收性能。用GPa中的弹性常数证实了其力学稳定性。由估计参数可知,随着压力的施加,脆性下降,在一定压力下,化合物具有延展性,这增加了其对光催化水分解应用的影响。在预期结果中观察到cscao3的各向异性。最后通过计算声子色散曲线和态密度来证实其动力学稳定性。根据我们的计算结果,我们的化合物具有良好的动态稳定性,这为将其用于光催化裂解水和析氢提供了特殊的优势。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


