Surface-immobilized cross-linking tetraalkylammonium cations networks mitigate hydrogen evolution for pure acidic CO2 reduction in proton-exchange membrane electrolyzers
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
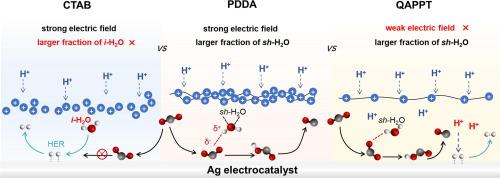
The scaling-up of electrochemical CO2 reduction requires circumventing the CO2 loss as carbonates under alkaline conditions. Zero-gap MEA cell configurations with a proton exchange membrane represent an alternative solution in a pure acidic system, but the catalyst layer in direct contact with the hydrated proton environment usually leads to H2 evolution dominating. Herein, we show that polydimethyldiallyl-ammonium-chloride-coated Ag (Ag@PDDA) electrode exhibits outstanding performance with a FE of 86 %, a single-pass conversion of 72 %, and a stability of 28 h for CO production in pure-acid MEA compared with ammonium poly(N-methyl-piperidine-co-pterphenyl) decorated Ag (Ag/QAPPT) and cetyltrimethylammonium bromide decorated Ag (Ag/CTAB). The in situ ATR-SEIRAS reveal that PDDA creates a positive charge-rich protective outer layer and an N-rich hybrid inner layer, which not only suppresses the migration of H+ during the electrolysis process and blocks the direct contact between H2O and Ag catalyst, but also promotes the generation from CO2 to *COOH in a pure-acid system. This work highlights the importance of polyelectrolyte engineering in regulating the electrocatalytic interface and accelerates the development of proton exchange membrane CO2 electrolysis.
在质子交换膜电解槽中,表面固定的交联四烷基铵阳离子网络减轻了纯酸性CO2还原的析氢
电化学CO2还原的扩大需要在碱性条件下规避CO2作为碳酸盐的损失。带有质子交换膜的零间隙MEA电池结构在纯酸性体系中是一种替代方案,但与水合质子环境直接接触的催化剂层通常导致H2的析出占主导地位。本文表明,与聚(n -甲基-吡啶- CO - terphenyl)修饰Ag (Ag/QAPPT)和十六烷基三甲基溴化铵修饰Ag (Ag/CTAB)相比,聚二甲基二烯丙基-氯化铵包覆Ag (Ag@PDDA)电极在纯酸MEA中表现出出色的性能,其FE为86%,单次转化率为72%,28 h的CO生成稳定性。原位ATR-SEIRAS表明,PDDA形成了富正电荷的保护外层和富n的杂化内层,不仅抑制了电解过程中H+的迁移,阻断了H2O与Ag催化剂的直接接触,而且促进了纯酸体系中CO2向*COOH的生成。这项工作突出了聚电解质工程在调节电催化界面中的重要性,加速了质子交换膜CO2电解的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


