Alloying-driven 3d orbital charge transfer for enhanced polysulfide adsorption and conversion in room temperature sodium-sulfur batteries
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
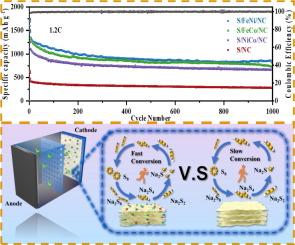
The severe shuttle effect and sluggish reaction kinetics in room-temperature sodium-sulfur (RT Na-S) batteries have been major bottlenecks hindering their practical application. To overcome these challenges, a straightforward reduction approach was employed to design three bimetallic alloy nanoparticles (FeNi, FeCo, and NiCo) supported on multistage porous carbon substrates. Experimental and theoretical calculations reveal that the charge transfer within the alloy catalyst influences the position of its d-band center and its degree of hybridization with sodium polysulfides (NaPSs). An increased charge transfer leads to a shift of the alloy’s d-band center closer to the Fermi energy level, thereby enhancing its adsorption and catalytic capabilities. Among the three alloy compositions, the FeNi alloy exhibits the highest charge transfer. Consequently, the FeNi alloy demonstrates the superior electrochemical performance, achieving a high reversible specific capacity of 848.2 mA h g−1, with an average capacity degradation rate of only 0.037 % per cycle over 1000 cycles at 1.2 C. The S/FeNi/NC cathode exhibits a low electrolyte-to-sulfur (E/S) ratio of 6.6 µL mg−1, while maintaining a high reversible specific capacity of 568.1 mA h g−1. This offers valuable insights for the application of alloy catalysts in the S/FeNi/NC cathode of RT Na-S batteries.
室温钠硫电池中增强多硫化物吸附和转化的合金驱动三维轨道电荷转移
室温钠硫(RT Na-S)电池的穿梭效应严重、反应动力学缓慢是制约其实际应用的主要瓶颈。为了克服这些挑战,采用直接还原方法设计了三种双金属合金纳米颗粒(FeNi, FeCo和NiCo),这些纳米颗粒支撑在多级多孔碳衬底上。实验和理论计算表明,合金催化剂内部的电荷转移影响其d带中心的位置及其与多硫化钠(NaPSs)的杂化程度。电荷转移的增加导致合金的d带中心向费米能级移动,从而增强其吸附和催化能力。在三种合金成分中,FeNi合金表现出最高的电荷转移。因此,FeNi合金表现出优异的电化学性能,在1.2℃下,在1000次循环中,其可逆比容量达到848.2 mA h g−1,平均容量衰减率仅为0.037%。S/FeNi/NC阴极的E/S比低至6.6 μ L mg−1,同时保持了568.1 mA h g−1的高可逆比容量。这为合金催化剂在RT Na-S电池S/FeNi/NC阴极中的应用提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


