Self-assembly of bortezomib nanofibers for solid tumor and bone metastasis therapy
IF 7.9
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
To overcome challenges including insufficient drug loading capacity, limited targeting accuracy, and the complex preparation of conventional nanomedicine, self-assembled nanomaterials have emerged as a viable solution. To explore the peptide self-assembly theory and overcome limitations, this study used bortezomib (BTZ) as the base material, and a novel peptide self-assembly strategy utilizing Zn(II) coordination was employed to prepare cancer cell-targeting nanofiber drugs (cRGD-BTZNDs). The therapeutic efficacy was evaluated in different types of tumors. The results demonstrated that cRGD-BTZNDs effectively entered cancer cells and exhibited enhanced cytotoxic effects against cancer cells compared to BTZ. Moreover, cRGD-BTZNDs exhibited excellent therapeutic efficacy against solid tumors, significantly inhibiting 4 T1 tumor growth while reducing biological toxicity. Additionally, in the treatment of bone metastases, cRGD-BTZNDs demonstrated excellent therapeutic potency, effectively alleviating bone damage in mice with high biocompatibility. This study not only self-assembled nanomaterials with great potential in cancer therapy, but also affirmed the correctness and universality of the Zn(II) coordination peptide self-assembly theory, providing a theoretical basis for the improvement of peptide-based nanomedicine.
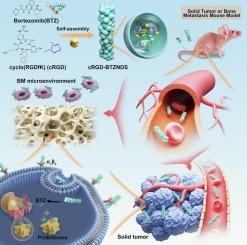
硼替佐米纳米纤维自组装用于实体瘤和骨转移治疗
为了克服载药能力不足、靶向精度有限以及传统纳米药物制备复杂等挑战,自组装纳米材料已经成为一种可行的解决方案。为了探索多肽自组装理论,克服局限性,本研究以硼替佐米(BTZ)为基础材料,采用一种利用Zn(II)配位的新型多肽自组装策略制备了靶向癌细胞的纳米纤维药物(cRGD-BTZNDs)。对不同类型肿瘤进行疗效评价。结果表明,与BTZ相比,cRGD-BTZNDs能有效进入癌细胞,并表现出更强的细胞毒作用。此外,cRGD-BTZNDs对实体瘤表现出良好的治疗效果,可显著抑制4 T1肿瘤生长,同时降低生物毒性。此外,在骨转移治疗中,cRGD-BTZNDs表现出优异的治疗效力,可有效减轻小鼠骨损伤,具有较高的生物相容性。本研究不仅证明了自组装纳米材料在癌症治疗中的巨大潜力,而且肯定了锌(II)配位肽自组装理论的正确性和普遍性,为改进基于肽的纳米医学提供了理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Materials & Design
Engineering-Mechanical Engineering
CiteScore
14.30
自引率
7.10%
发文量
1028
审稿时长
85 days
期刊介绍:
Materials and Design is a multi-disciplinary journal that publishes original research reports, review articles, and express communications. The journal focuses on studying the structure and properties of inorganic and organic materials, advancements in synthesis, processing, characterization, and testing, the design of materials and engineering systems, and their applications in technology. It aims to bring together various aspects of materials science, engineering, physics, and chemistry.
The journal explores themes ranging from materials to design and aims to reveal the connections between natural and artificial materials, as well as experiment and modeling. Manuscripts submitted to Materials and Design should contain elements of discovery and surprise, as they often contribute new insights into the architecture and function of matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


