3D-printed barriers with machine learning powered image analysis for enhanced wound healing assays
IF 7.9
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
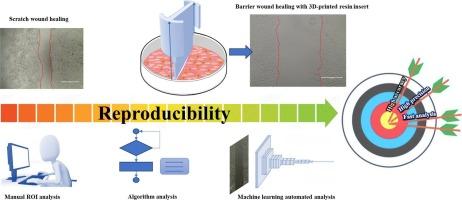
Wound healing assay is a standard method enabling investigation of cell proliferation and migration through a cell-free gap in a cell monolayer. Despite very common, it shows several weaknesses: lack of reproducibility and manual and time-based image analysis. Based on novel approach founded on innovative materials and AI-assisted processing of biological images, a promising automated barrier-wound healing assay is realized, achieving consistent results while retaining cells integrity. To increase assay accuracy, biocompatible 3D-printed resin inserts have been developed, facilitating precise control over shape and size of the wound. In parallel, a new image-detection algorithm powered by Deep Learning models was developed to identify cell-free area during the healing process, exceeding limitations of manual analysis. 3D-resin inserts combined with automated image analysis allowed the elimination of subjective errors and provided reproducible quantification of cell-free areas across multiple experiments.
Moreover, a dataset to train a Convolutional Neural Network for monitor healing over time was developed.
As proof of concept, this algorithm was tested on a cancer cell line stimulated by TGF-β, a drug stimulating cell migration.
Innovative design of biocompatible materials combined with Deep Learning for automatically processing high-throughput data enables standardized wound healing assay, increasing efficiency, reliability, and accuracy of results.
3d打印屏障与机器学习驱动的图像分析,增强伤口愈合分析
伤口愈合试验是一种标准的方法,可以通过细胞单层中的无细胞间隙来研究细胞增殖和迁移。尽管非常普遍,但它显示出几个缺点:缺乏再现性和手动和基于时间的图像分析。基于创新材料和人工智能辅助生物图像处理的新方法,实现了一种有前途的自动化屏障伤口愈合试验,在保持细胞完整性的同时获得一致的结果。为了提高检测准确性,生物相容性3d打印树脂插入物已经开发出来,有助于精确控制伤口的形状和大小。与此同时,一种由深度学习模型驱动的新的图像检测算法被开发出来,用于识别愈合过程中的无细胞区域,超越了人工分析的限制。3d树脂插入与自动图像分析相结合,可以消除主观误差,并在多个实验中提供可重复的无细胞区域定量。此外,还开发了一个用于训练卷积神经网络的数据集,用于监测随时间推移的愈合情况。作为概念验证,该算法在TGF-β(一种刺激细胞迁移的药物)刺激的癌细胞系上进行了测试。生物相容性材料的创新设计与深度学习相结合,可自动处理高通量数据,实现标准化伤口愈合分析,提高效率,可靠性和结果的准确性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Materials & Design
Engineering-Mechanical Engineering
CiteScore
14.30
自引率
7.10%
发文量
1028
审稿时长
85 days
期刊介绍:
Materials and Design is a multi-disciplinary journal that publishes original research reports, review articles, and express communications. The journal focuses on studying the structure and properties of inorganic and organic materials, advancements in synthesis, processing, characterization, and testing, the design of materials and engineering systems, and their applications in technology. It aims to bring together various aspects of materials science, engineering, physics, and chemistry.
The journal explores themes ranging from materials to design and aims to reveal the connections between natural and artificial materials, as well as experiment and modeling. Manuscripts submitted to Materials and Design should contain elements of discovery and surprise, as they often contribute new insights into the architecture and function of matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


