Medium-entropy-induced in-situ surface spinel phase towards stable Co-free Li-rich cathode material
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
Co-free Li-rich Li1.2Ni0.2Mn0.6O2 (LR) cathode shows the highest working capacity that can be applied to high-energy density Li-ion batteries (LIBs). However, poor cycle stability and voltage decay caused by phase transition are always hindering its further development. Herein, a novel medium-entropy Li-rich Mn-based cathode material (LRMEF) was synthesized via a simple sol-gel method. The introduction of multivalent ions (Al3+/Cu2+ doping at Mn sites and F− doping at O sites) effectively mitigates the Jahn-Teller distortion of Mn ions and suppresses oxygen release. High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images confirm that this synergistic doping strategy induces the in-situ formation of an approximately 3 nm-thick spinel surface layer, which significantly enhances structural stability and ion diffusion kinetics. Besides, a series of in-situ/ex-situ characterization methods and density functional theory (DFT) calculations have been carried out to fundamentally shed light on the optimized structure-activity relationship and reaction mechanism. As a result, the LR material with entropy regulation and anion doping exhibits excellent cycling stability (189.2 mAh g−1 at 1 C with 84 % capacity retention after 300 cycles), rate performance (164.1 mAh g−1 at 5 C), and voltage retention (82.7 % at 1 C after 300 cycles), demonstrating great application prospects in future high-energy-density LIBs.
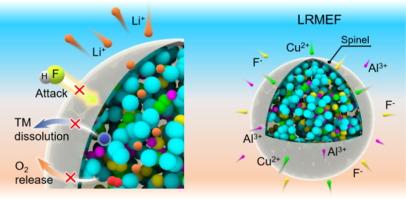
中熵诱导原位尖晶石相形成稳定的无钴富锂正极材料
无co -rich li - Li1.2Ni0.2Mn0.6O2 (LR)阴极具有最高的工作容量,可应用于高能密度锂离子电池(LIBs)。然而,周期稳定性差和相变引起的电压衰减一直阻碍着其进一步发展。本文采用简单的溶胶-凝胶法制备了一种新型中熵富锂锰基正极材料。多价离子的引入(Al3+/Cu2+掺杂在Mn位点,F -掺杂在O位点)有效地减轻了Mn离子的Jahn-Teller畸变,抑制了氧的释放。高角度环形暗场扫描透射电子显微镜(HAADF-STEM)图像证实,这种协同掺杂策略诱导原位形成约3nm厚的尖晶石表面层,显著提高了结构稳定性和离子扩散动力学。此外,通过一系列原位/非原位表征方法和密度泛函理论(DFT)计算,从根本上揭示了优化后的构效关系和反应机理。结果表明,经过熵调节和阴离子掺杂的LR材料具有优异的循环稳定性(1℃下189.2 mAh g−1,300次循环后容量保持率84%)、倍率性能(5℃下164.1 mAh g−1)和电压保持率(300次循环后1℃下82.7%),在未来高能量密度锂离子电池中具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


