Enhanced mechanical strength and improved Li+ transport in PEO-based electrolytes via scalable bicontinuous PMIA porous membrane
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
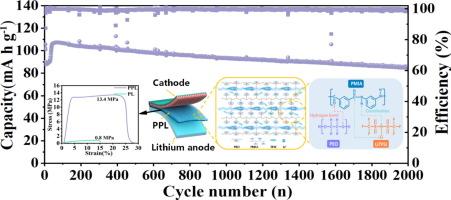
The low ionic conductivity and poor mechanical strength of polyethylene oxide (PEO)-based electrolytes severely restrict their practical application. To address this problem, this work designs a scalable, high-strength (24.3 MPa) bicontinuous porous poly (m-phthaloyl-m-phenylenediamine) (PMIA) membrane integrated into PEO/LiTFSI (PL), thus forming a PMIA/PEO/LiTFSI (PPL) composite electrolyte. Compared to the PL electrolyte, the PPL electrolyte reinforced by a bicontinuous porous PMIA membrane exhibits significantly enhanced mechanical strength, reaching 13.4 MPa. In addition, the amide groups on PMIA strongly coordinate with LiTFSI and form hydrogen bonds with PEO, promoting Li salt dissociation and reducing the Li+ migration barrier. This creates efficient, fast Li+ transport channels at the PMIA/PL interfaces, effectively promoting the uniform Li+ deposition and minimizing lithium dendrite formation. The PPL electrolyte achieves high ionic conductivity (1×10−4 S cm−1 at 30 °C) and Li+ transference number (tLi+=0.43). The assembled LiFePO4/Li battery demonstrates excellent cycling stability, retaining 80% capacity after 2000 cycles at 2 C, while the Li/Li symmetric cell operates stably for over 900 h at 0.3 mA cm−2. Therefore, the scalable porous PMIA membrane effectively enhances both the mechanical strength and Li+ transport in PEO-based electrolytes, offering a viable strategy for their commercial-scale implementation.
通过可伸缩双连续PMIA多孔膜增强peo基电解质的机械强度并改善Li+输运
聚乙烯氧化物(PEO)电解质离子电导率低,机械强度差,严重制约了其实际应用。为了解决这一问题,本研究设计了一种可扩展的,高强度(24.3 MPa)双连续多孔聚(间苯二胺-间苯二胺)(PMIA)膜集成到PEO/LiTFSI (PL)中,从而形成PMIA/PEO/LiTFSI (PPL)复合电解质。与PL电解质相比,双连续多孔PMIA膜增强PPL电解质的机械强度显著提高,达到13.4 MPa。此外,PMIA上的酰胺基团与LiTFSI强配位,与PEO形成氢键,促进Li盐解离,降低Li+迁移屏障。这在PMIA/PL界面上创造了高效、快速的Li+传输通道,有效地促进了Li+的均匀沉积,并最大限度地减少了锂枝晶的形成。PPL电解质具有较高的离子电导率(1×10−4 S cm−1,30℃)和Li+转移数(tLi+=0.43)。组装的LiFePO4/Li电池表现出优异的循环稳定性,在2℃下循环2000次后仍保持80%的容量,而Li/Li对称电池在0.3 mA cm−2下稳定工作900小时以上。因此,可伸缩多孔PMIA膜有效地提高了peo基电解质的机械强度和Li+输运,为其商业规模的实施提供了可行的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


