Dual-regulation tailoring of tunnel-structured hexagonal tungsten oxide for high-performance ammonium-ion hybrid supercapacitors
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
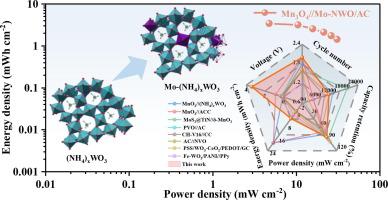
Ammonium-ion hybrid supercapacitors (A-HSCs) have emerged as promising candidates for next-generation energy storage owing to their inherent safety and environmental sustainability. Hexagonal tungsten oxide (h-WO3), with its well-defined tunnel structure, holds great promise as a negative electrode material for NH4+ storage. However, its practical application is hindered by structural instability and poor intrinsic electrical conductivity. To address these challenges, a dual-regulation strategy is proposed, integrating molybdenum (Mo) doping and NH4+ pre-intercalation to concurrently optimize the tunnel structure and electronic environment of h-WO3 (Mo-NWO). Comprehensive experimental and theoretical analyses reveal that Mo doping narrows the bandgap of WO3 and reduces the diffusion energy barrier, thereby accelerating NH4+ adsorption and diffusion. Simultaneously, NH4+ pre-intercalation stabilizes the tunnel framework via hydrogen bonding, ensuring structural reversibility. As expected, the Mo-NWO/AC electrode achieves a high areal capacitance of 13.6 F cm−2 at 5 mA cm−2 and retains 80.14 % of its capacitance after 5000 cycles, demonstrating exceptional rate capability and cycling stability. Moreover, the assembled Mn3O4//Mo-NWO/AC device delivers a high energy density of 3.41 mWh cm−2 and outstanding long-term stability (85.75 % retention after 12,000 cycles). This work provides a viable strategy for designing high-performance NH4+ storage materials and advances the development of sustainable energy storage systems.
用于高性能铵离子混合超级电容器的隧道结构六方氧化钨双调节裁剪
氨离子混合超级电容器(a - hsc)由于其固有的安全性和环境可持续性而成为下一代储能的有希望的候选者。六方氧化钨(h-WO3)具有良好的隧道结构,有望作为NH4+的负极材料。然而,它的实际应用受到结构不稳定和固有导电性差的阻碍。为了解决这些问题,提出了一种双调控策略,结合钼(Mo)掺杂和NH4+预插层,同时优化h-WO3 (Mo- nwo)的隧道结构和电子环境。综合实验和理论分析表明,Mo掺杂缩小了WO3的带隙,降低了扩散能垒,从而加速了NH4+的吸附和扩散。同时,NH4+预插层通过氢键稳定了隧道框架,保证了结构的可逆性。正如预期的那样,Mo-NWO/AC电极在5 mA cm - 2下实现了13.6 F cm - 2的高面电容,并且在5000次循环后保持了80.14%的电容,表现出卓越的速率能力和循环稳定性。此外,组装的Mn3O4//Mo-NWO/AC器件具有3.41 mWh cm - 2的高能量密度和出色的长期稳定性(12,000次循环后保持率为85.75%)。该研究为设计高性能NH4+储能材料提供了可行的策略,并推动了可持续储能系统的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


