Diverse synthesis of bridged bicyclo[3.2.1]octa-2,6-diene and tricyclo[3.2.1.02,7] oct-3-ene frameworks via stepwise cascade reactions
IF 8.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Strained bridged rings bicyclo[3.2.1]octane and tricyclo[3.2.1.02,7]octane are prevalent in natural products known for their significant biological activities. However, strategies for efficiently synthesizing these complex frameworks from simple starting materials via de novo synthesis remain underexplored. This article presents an efficient strategy that combines phosphine catalysis and photocatalysis to execute a stepwise tandem reaction involving allenoates and α-cyano cinnamaldehydes, including [3 + 2] cyclization, [5 + 2] cyclization, acyl transfer, and decarboxylation reactions, synthesizing a series of functional bicyclo[3.2.1]octa-2,6-diene and tricyclo[3.2.1.02,7]oct‑3-ene skeleton derivatives with excellent chemoselectivity demonstrated throughout the process. Meanwhile, the reaction can also be performed via a one-pot, scalable phosphine/photocatalytic cascade process, efficiently yielding the bridged products which can serve as versatile intermediates for further applications.
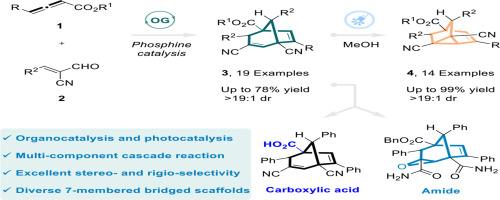
阶梯级联反应合成双环[3.2.1]辛-2,6-二烯和三环[3.2.1.02,7]辛-3-烯框架
张力桥环双环[3.2.1]辛烷和三环[3.2.1.02,7]辛烷因其重要的生物活性而普遍存在于天然产物中。然而,通过从头合成从简单的起始材料有效合成这些复杂框架的策略仍未得到充分探索。本文提出了一种结合磷化氢催化和光催化的高效策略,通过[3 + 2]环化、[5 + 2]环化、酰基转移和脱羧反应,进行了烯丙酸盐与α-氰基肉桂醛的阶梯串联反应,合成了一系列具有良好化学选择性的双环[3.2.1]辛二烯-2,6-二烯和三环[3.2.1.02,7]oct - 3-烯骨架衍生物。同时,该反应也可以通过一锅,可扩展的磷化氢/光催化级联过程进行,有效地产生桥接产物,可以作为多功能中间体用于进一步应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


