Human iPSC-derived cardiac-specific extracellular matrix scaffolds for cardiomyocyte maturation and post-myocardial infarction repair
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
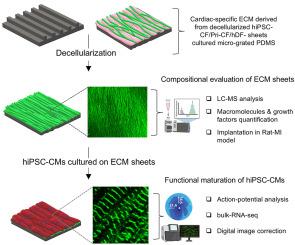
Myocardial infarction (MI) remains a leading cause of heart failure due to the limited regenerative capacity of the adult myocardium. The therapeutic efficacy of current engineered cardiac patches is hindered by their simplistic scaffold composition and lack of structural organization. This study presents a bioactive, anisotropic extracellular matrix (ECM) scaffold derived from human induced pluripotent stem cell-differentiated cardiac fibroblasts (hiPSC-CF-ECM) that combines cardiac-specific proteins and growth factors with complex structural composition. Compared to primary cardiac fibroblast ECM (pri-CF-ECM) and human dermal fibroblast ECM (hDF-ECM), hiPSC-derived cardiomyocytes (hiPSC-CMs) cultured on the cardiac-specific ECM scaffold exhibited enhanced maturation, as confirmed by bulk RNA sequencing, electrophysiological mapping, and optical-based strain analysis. In an immune-competent rat MI model, the hiPSC-CF-ECM transplantation preserved cardiac function, increased ejection fraction, and reduced maladaptive remodeling. These findings highlight hiPSC-CF-ECM as a promising biomimetic scaffold for cardiac tissue engineering and MI treatment.
人类ipsc衍生的心脏特异性细胞外基质支架用于心肌细胞成熟和心肌梗死后修复
由于成人心肌的再生能力有限,心肌梗死(MI)仍然是心力衰竭的主要原因。目前工程化心脏贴片的治疗效果受到支架组成简单和缺乏结构组织的阻碍。本研究提出了一种生物活性、各向异性的细胞外基质(ECM)支架,该支架来源于人诱导多能干细胞分化的心脏成纤维细胞(hiPSC-CF-ECM),该支架结合了具有复杂结构成分的心脏特异性蛋白质和生长因子。与原代心脏成纤维细胞ECM (pri-CF-ECM)和人真皮成纤维细胞ECM (hDF-ECM)相比,在心脏特异性ECM支架上培养的hipsc来源的心肌细胞(hiPSC-CMs)表现出更强的成熟,这一点得到了大量RNA测序、电生理定位和基于光学的strain分析的证实。在免疫能力大鼠心肌梗死模型中,hiPSC-CF-ECM移植保留了心功能,增加了射血分数,减少了不适应重构。这些发现突出了hiPSC-CF-ECM作为心脏组织工程和心肌梗死治疗的仿生支架的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


