Haplotype-resolved genomics identifies cyp19a1a as a candidate master sex-determining gene in golden trevally (Gnathanodon speciosus)
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
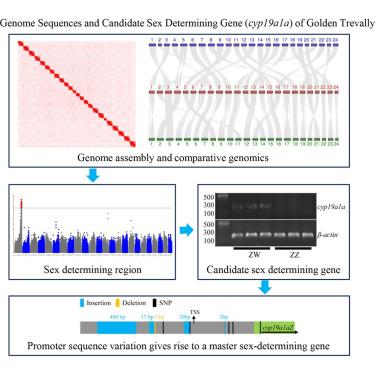
Teleost fish exhibit diverse genetic sex determination systems, offering opportunities to understand its genetic basis and insights into sex chromosome evolution. The golden trevally (Gnathanodon speciosus), a commercially important Carangidae species, was studied to uncover its sex determination mechanism. We generated haplotype-resolved, chromosome-level genome assemblies for both sexes and identified a ZW system with a ∼90 kb sex-determining region (SDR) on Chr1. Within the SDR, cyp19a1a is uniquely expressed from the W allele prior to gonadal differentiation. A ∼1-kb upstream region of cyp19a1a harbors fixed variants between sexes. Reporter gene assays reveal that these variants reduce promoter activity in the Z allele, likely silencing cyp19a1aZ. This suggests that allelic diversification led to the emergence of cyp19a1aW as the master sex-determining gene. Minimal divergence in the SDR implies a recent origin of sex chromosomes. These findings provide key genomic resources and insights into sex determination evolution in teleosts.
单倍型解析基因组学鉴定cyp19a1a为金齿鲨(齿鲨)的候选主性别决定基因。
硬骨鱼表现出不同的遗传性别决定系统,为了解其遗传基础和洞察性染色体进化提供了机会。本文研究了一种具有重要商业价值的金鲹(Gnathanodon speciosus)的性别决定机制。我们生成了单倍型解析的染色体水平的两性基因组组合,并鉴定了一个在Chr1上具有约90 kb性别决定区(SDR)的ZW系统。在SDR中,cyp19a1a在性腺分化之前由W等位基因唯一表达。cyp19a1a上游约1-kb的区域包含两性之间的固定变异。报告基因分析显示,这些变异降低了Z等位基因的启动子活性,可能沉默了cyp19a1aZ。这表明等位基因多样化导致了cyp19a1aW作为主要性别决定基因的出现。SDR的最小分化暗示了性染色体最近的起源。这些发现为硬骨鱼的性别决定进化提供了关键的基因组资源和见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


