Sex-specific expression and function of TRIM28 during mouse primordial germ differentiation
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Mammalian primordial germ cells (PGCs) are embryonic precursors to the adult germline and must facilitate high-fidelity transfer of genomic material from one generation to the next. Transposable elements (TEs) represent an ongoing threat to genomic fidelity and are therefore tightly controlled during embryonic germline development. Here, we find that some TEs change in accessibility during normal PGC differentiation, while others are constitutively repressed by tripartite motif-containing 28 (TRIM28), a master TE regulator. We find that TRIM28 itself is regulated in a sex-specific manner and represses sex-specific TEs. In both testicular and ovarian PGCs, TRIM28 protects against upregulation of 2-cell (2C)-associated genes, dysregulation of PGC differentiation, and incomplete activation of DAZL. This perturbs testicular and ovarian PGCs differently, with testicular PGCs failing to differentiate in embryonic life while ovarian PGCs inefficiently enter meiosis leading to a diminished ovarian reserve by the onset of sexual maturity.
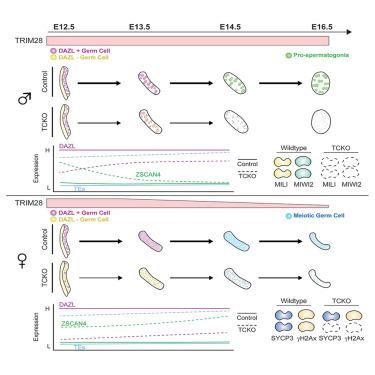
TRIM28在小鼠原始生殖分化过程中的性别特异性表达和功能
哺乳动物原始生殖细胞(PGCs)是成年生殖系的胚胎前体,必须促进基因组物质从一代到下一代的高保真转移。转座因子(te)是对基因组保真度的持续威胁,因此在胚胎种系发育过程中受到严格控制。在这里,我们发现在正常的PGC分化过程中,一些TE的可达性发生了变化,而另一些则被tripartite motif-containing 28 (TRIM28)组成性抑制,TRIM28是一个主要的TE调节因子。我们发现TRIM28本身以性别特异性的方式调节,并抑制性别特异性的TEs。在睾丸和卵巢PGCs中,TRIM28可以防止2-细胞(2C)相关基因的上调、PGC分化的失调和DAZL的不完全激活。这对睾丸和卵巢PGCs的干扰不同,睾丸PGCs在胚胎期不能分化,而卵巢PGCs不能有效地进入减数分裂,导致性成熟开始时卵巢储备减少。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


