Bone matrix-mimetic multi-ion doped hydroxyapatite nanorod arrays for enhanced immuno-osteogenesis and prevention of aseptic loosening
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Inflammatory responses triggered by foreign body rejection or detached wear particles are major contributors to the failure of titanium (Ti)-based orthopedic implants. One of the most common complications is aseptic loosening. To address this challenge, a nanorod-arrayed hydroxyapatite (HA) coating co-doped with multiple ions (Sr2+, Mg2+, Zn2+, CO32−, and SiO44−) was developed, to mimic the bone matrix and enhance immuno-osteogenesis. The coating exhibits strong adhesion strength and long-term interfacial stability. Ion-induced lattice distortion accelerates HA degradation, promoting the doped-ion release. These bioactive ions drive macrophage (MΦs) polarization from pro-inflammatory M1 to anti-inflammatory M2 phenotype, thereby enhancing vascularized osseointegration. To evaluate the risk of aseptic loosening induced by detached nanorods, we synthesized nanorod-like particles with identical composition and morphology to those in the arrayed coating. Their effects on MΦ polarization and the underlying mechanisms were then systematically investigated. Compared to undoped particles, multi-ion doped HA nanoparticles are more easily phagocytosed and degraded within lysosomes, enabling faster ion release and thereby facilitating M1-M2 transition. Transcriptomic analysis reveals that the nanoparticle degradation-mediated ion release elevates intracellular calcium levels in MΦs, which activates antioxidant pathways and induces a metabolic shift toward fatty acid oxidation. This metabolic reprogramming subsequently activates immunoregulatory pathways, with the PPAR pathway serving as a central axis, thereby promoting M2 polarization, inhibiting osteoclastogenesis, and ultimately preventing aseptic loosening. Collectively, this study presents a coating design that not only promotes osseointegration but also retains anti-inflammatory potential even in the event of particle detachment, offering a promising strategy to mitigate aseptic loosening.
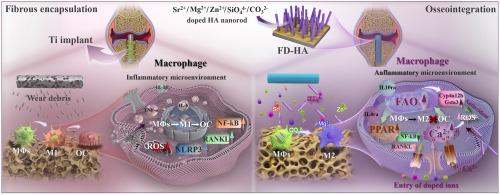
模拟骨基质多离子掺杂羟基磷灰石纳米棒阵列增强免疫成骨和预防无菌性松动
由异物排斥反应或脱落磨损颗粒引发的炎症反应是钛基骨科植入物失败的主要原因。最常见的并发症之一是无菌性松动。为了解决这一挑战,研究人员开发了一种纳米棒排列的羟基磷灰石(HA)涂层,共掺杂多种离子(Sr2+, Mg2+, Zn2+, CO32−和SiO44−),以模拟骨基质并增强免疫成骨。该涂层具有较强的附着力和长期的界面稳定性。离子诱导的晶格畸变加速了HA的降解,促进了掺杂离子的释放。这些生物活性离子驱动巨噬细胞(MΦs)从促炎M1型向抗炎M2型极化,从而增强血管化骨整合。为了评估分离的纳米棒引起无菌性松动的风险,我们合成了与阵列涂层中纳米棒具有相同组成和形态的纳米棒状颗粒。然后系统地研究了它们对MΦ极化的影响及其潜在机制。与未掺杂的粒子相比,多离子掺杂的HA纳米粒子更容易在溶酶体内被吞噬和降解,从而使离子释放更快,从而促进M1-M2的转变。转录组学分析显示,纳米颗粒降解介导的离子释放提高MΦs细胞内钙水平,从而激活抗氧化途径并诱导代谢向脂肪酸氧化转变。这种代谢重编程随后激活免疫调节途径,以PPAR途径为中心轴,从而促进M2极化,抑制破骨细胞发生,最终防止无菌性松动。总的来说,这项研究提出了一种涂层设计,不仅可以促进骨整合,而且即使在颗粒脱离的情况下也能保持抗炎潜力,为减轻无菌性松动提供了一种有希望的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


