An intelligent nanoreservoir system promotes heart valve regeneration via ROS scavenging and immunomodulation
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
In situ regeneration of tissue-engineered heart valves (TEHV) is a promising strategy to overcome the limitations of existing heart valve prostheses. Although, decellularized aortic valves (DAVs) are widely regarded as a scaffold in the construction of TEHV, the poor hemocompatibility and adverse immune responses make DAV scaffolds prone to thrombosis and degradation, thus hindering recellularization and in situ regeneration. Our study developed an immune-regulatory strategy involving the use of hydrogel-encapsulated nanoparticles to modify DAV scaffolds. Specifically, folic acid- and hyaluronic acid-modified mesoporous silica nanoparticles (FA-HA-MSNs@CY-09) were engineered to deliver NOD-like receptor family, pyrin domain containing 3 (NLRP3) inhibitor CY-09, thereby targeting macrophages, modulating their polarization and establishing a pro-regenerative immune microenvironment. The reactive oxygen species (ROS)-responsive hydrogel delivering FA-HA-MSNs@CY-09 enabled intelligent nanoparticle release and ROS scavenging. Results demonstrated that the hydrogel-modified DAV scaffold exhibited ROS-responsive release of FA-HA-MSNs@CY-09, which effectively induced macrophage polarization toward the pro-remodeling M2 phenotype. In vitro, the scaffold showed favorable mechanical properties, cytocompatibility, and hemocompatibility. Transcriptome sequencing elucidated the macrophage-reprogramming mechanism of the scaffold. In vivo, the scaffolds promoted significant M2 macrophage infiltration shortly after implantation, facilitating endothelial tissue formation. This resulted in enhanced endothelialization and interstitial cell infiltration under blood flow, without thrombosis or calcification. The novel heart valve overcomes various limitations of conventional heart valve prostheses and demonstrates considerable promise for clinical translation, particularly as an immunomodulatory biomaterial strategy.
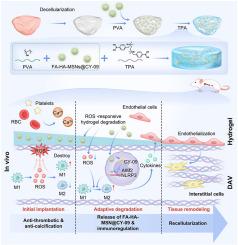
智能纳米储存库系统通过活性氧清除和免疫调节促进心脏瓣膜再生
组织工程心脏瓣膜原位再生(TEHV)是一种很有前途的策略,可以克服现有心脏瓣膜假体的局限性。尽管脱细胞主动脉瓣(DAVs)被广泛认为是构建tev的支架,但血液相容性差和不良的免疫反应使得DAV支架容易形成血栓和降解,从而阻碍了再细胞化和原位再生。我们的研究开发了一种免疫调节策略,包括使用水凝胶封装的纳米颗粒来修饰DAV支架。具体来说,叶酸和透明质酸修饰的介孔二氧化硅纳米颗粒(FA-HA-MSNs@CY-09)被设计用于递送nod样受体家族,pyrin结构域含有3 (NLRP3)抑制剂CY-09,从而靶向巨噬细胞,调节其极化并建立促进再生的免疫微环境。活性氧(ROS)响应水凝胶递送FA-HA-MSNs@CY-09实现智能纳米颗粒释放和ROS清除。结果表明,水凝胶修饰的DAV支架具有ros响应性释放FA-HA-MSNs@CY-09,可有效诱导巨噬细胞向促重塑M2表型极化。体外支架表现出良好的力学性能、细胞相容性和血液相容性。转录组测序阐明了支架的巨噬细胞重编程机制。在体内,支架在植入后不久促进了M2巨噬细胞的显著浸润,促进了内皮组织的形成。这导致血流下内皮化和间质细胞浸润增强,没有血栓形成或钙化。这种新型心脏瓣膜克服了传统心脏瓣膜假体的各种局限性,并在临床转化方面表现出相当大的前景,特别是作为一种免疫调节生物材料策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


